Abstract
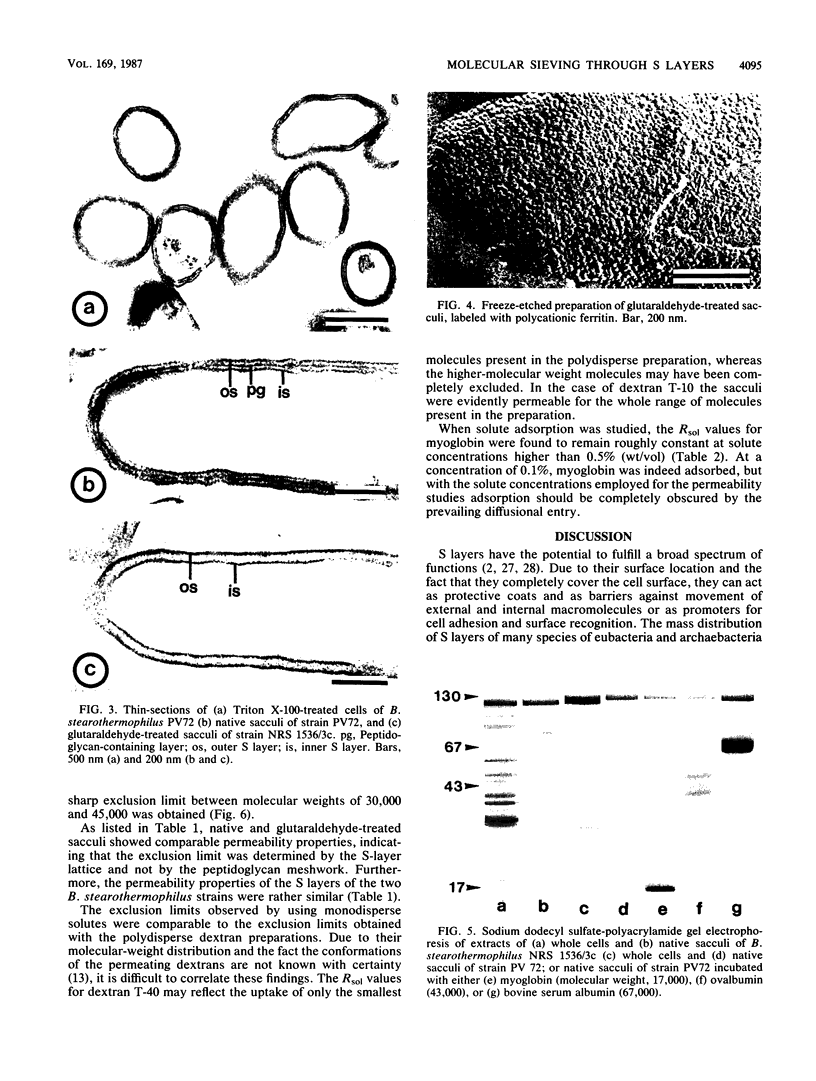
The permeability properties and the exclusion limits of the crystalline surface layers (S layers) of two selected strains of Bacillus stearothermophilus were investigated. Measurements were performed of passive solute uptake into the intracellular space of native or glutaraldehyde-treated sacculi. Native sacculi were prepared from whole cells by extracting the cytoplasmic membrane with Triton X-100 under conditions which preserved the integrity of the S layer and the peptidoglycan-containing layer. The permeability barrier was found to consist of three adjacent layers, namely, the S layer, the peptidoglycan-containing layer, and an incomplete S layer attached to the inner face of the peptidoglycan-containing layer. In glutaraldehyde-treated sacculi the peptidoglycan was digested after stabilizing the S-layer lattice by chemical cross-linking. The solutes selected for the uptake measurements were mannose, proteins, and dextrans of increasing molecular weights. The S layers of both strains allowed free passage for molecules with a molecular weight of up to 30,000 and showed sharp exclusion limits between molecular weights of 30,000 and 45,000, suggesting a limiting pore diameter of about 4.5 nm.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Smith P. R., Dubochet J., Henry C., Kellenberger E. A study of the structure of the T-layer of Bacillus brevis. J Supramol Struct. 1973;1(6):498–522. doi: 10.1002/jss.400010606. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- CONWAY E. J., DOWNEY M. An outer metabolic region of the yeast cell. Biochem J. 1950 Sep;47(3):347–355. doi: 10.1042/bj0470347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon D., Goldstein L., Marikovsky Y., Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972 Mar;38(5):500–510. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Deatherage J. F., Taylor K. A., Amos L. A. Three-dimensional arrangement of the cell wall protein of Sulfolobus acidocaldarius. J Mol Biol. 1983 Jul 15;167(4):823–848. doi: 10.1016/s0022-2836(83)80113-2. [DOI] [PubMed] [Google Scholar]
- Dickson M. R., Downing K. H., Wu W. H., Glaeser R. M. Three-dimensional structure of the surface layer protein of Aquaspirillum serpens VHA determined by electron crystallography. J Bacteriol. 1986 Sep;167(3):1025–1034. doi: 10.1128/jb.167.3.1025-1034.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt H., Saxton W. O., Baumeister W. Three-dimensional structure of the tetragonal surface layer of Sporosarcina ureae. J Bacteriol. 1986 Oct;168(1):309–317. doi: 10.1128/jb.168.1.309-317.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCOIS C., MARSHALL R. D., NEUBERGER A. Carbohydrates in protein. 4. The determination of mannose in hen's-egg albumin by radioisotope dilution. Biochem J. 1962 May;83:335–341. doi: 10.1042/bj0830335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROTTE G. Passage of dextran molecules across the blood-lymph barrier. Acta Chir Scand Suppl. 1956;211:1–84. [PubMed] [Google Scholar]
- Glaeser R. M., Chiu W., Grano D. Structure of the surface layer protein of the outer membrane of Spirillum serpens. J Ultrastruct Res. 1979 Mar;66(3):235–242. doi: 10.1016/s0022-5320(79)90121-7. [DOI] [PubMed] [Google Scholar]
- Glenn A. R. Production of extracellular proteins by bacteria. Annu Rev Microbiol. 1976;30:41–62. doi: 10.1146/annurev.mi.30.100176.000353. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Phipps B. M., Ishiguro E. E., Olafson R. W., Trust T. J. Surface layer virulence A-proteins from Aeromonas salmonicida strains. Can J Biochem Cell Biol. 1984 Nov;62(11):1064–1071. doi: 10.1139/o84-137. [DOI] [PubMed] [Google Scholar]
- Küpcü Z., März L., Messner P., Sleytr U. B. Evidence for the glycoprotein nature of the crystalline cell wall surface layer of Bacillus stearothermophilus strain NRS2004/3a. FEBS Lett. 1984 Jul 23;173(1):185–190. doi: 10.1016/0014-5793(84)81043-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lepault J., Martin N., Leonard K. Three-dimensional structure of the T-layer of Bacillus sphaericus P-1. J Bacteriol. 1986 Oct;168(1):303–308. doi: 10.1128/jb.168.1.303-308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner P., Pum D., Sára M., Stetter K. O., Sleytr U. B. Ultrastructure of the cell envelope of the archaebacteria Thermoproteus tenax and Thermoproteus neutrophilus. J Bacteriol. 1986 Jun;166(3):1046–1054. doi: 10.1128/jb.166.3.1046-1054.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V., Murray R. G. Ultrastructure of the cell wall of Bacillus polymyxa. J Bacteriol. 1967 Jun;93(6):1949–1965. doi: 10.1128/jb.93.6.1949-1965.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders R. L., May B. K. Evidence for extrusion of unfolded extracellular enzyme polypeptide chains through membranes of Bacillus amyloliquefaciens. J Bacteriol. 1975 Sep;123(3):806–814. doi: 10.1128/jb.123.3.806-814.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer R., Gerhardt P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J Bacteriol. 1971 Sep;107(3):718–735. doi: 10.1128/jb.107.3.718-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer R., Louden L., Gerhardt P. Porosity of the yeast cell wall and membrane. J Bacteriol. 1974 May;118(2):534–540. doi: 10.1128/jb.118.2.534-540.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren A., Hovmöller S., Farrants G., Ranta H., Haapasalo M., Ranta K., Lounatmaa K. Structures of two different surface layers found in six Bacteroides strains. J Bacteriol. 1985 Dec;164(3):1278–1282. doi: 10.1128/jb.164.3.1278-1282.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Sára M., Küpcü Z., Messner P. Structural and chemical characterization of S-layers of selected strains of Bacillus stearothermophilus and Desulfotomaculum nigrificans. Arch Microbiol. 1986 Oct;146(1):19–24. doi: 10.1007/BF00690152. [DOI] [PubMed] [Google Scholar]
- Squire P. G., Himmel M. E. Hydrodynamics and protein hydration. Arch Biochem Biophys. 1979 Aug;196(1):165–177. doi: 10.1016/0003-9861(79)90563-0. [DOI] [PubMed] [Google Scholar]
- Stewart M., Beveridge T. J., Murray R. G. Structure of the regular surface layer of Spirillum putridiconchylium. J Mol Biol. 1980 Feb 15;137(1):1–8. doi: 10.1016/0022-2836(80)90153-9. [DOI] [PubMed] [Google Scholar]
- Stewart M., Beveridge T. J. Structure of the regular surface layer of Sporosarcina ureae. J Bacteriol. 1980 Apr;142(1):302–309. doi: 10.1128/jb.142.1.302-309.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M., Murray R. G. Structure of the regular surface layer of Aquaspirillum serpens MW5. J Bacteriol. 1982 Apr;150(1):348–357. doi: 10.1128/jb.150.1.348-357.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sára M., Sleytr U. B. Charge distribution on the S layer of Bacillus stearothermophilus NRS 1536/3c and importance of charged groups for morphogenesis and function. J Bacteriol. 1987 Jun;169(6):2804–2809. doi: 10.1128/jb.169.6.2804-2809.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallinder I. B., Neujahr H. Y. CELL WALL AND PEPTIDOGLYCAN FROM Lactobacillus fermenti. J Bacteriol. 1971 Mar;105(3):918–926. doi: 10.1128/jb.105.3.918-926.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]






