Abstract
Protein phosphatase-1 (PP-1) is involved in the regulation of numerous metabolic processes in mammalian cells. The major isoforms of PP-1, α, γ1, and δ, have nearly identical catalytic domains, but they vary in sequence at their extreme NH2 and COOH termini. With specific antibodies raised against the unique COOH-terminal sequence of each isoform, we find that the three PP-1 isoforms are each expressed in all mammalian cells tested, but that they localize within these cells in a strikingly distinct and characteristic manner. Each isoform is present both within the cytoplasm and in the nucleus during interphase. Within the nucleus, PP-1 α associates with the nuclear matrix, PP-1 γ1 concentrates in nucleoli in association with RNA, and PP-1 δ localizes to nonnucleolar whole chromatin. During mitosis, PP-1 α is localized to the centrosome, PP-1 γ1 is associated with microtubules of the mitotic spindle, and PP-1 δ strongly associates with chromosomes. We conclude that PP-1 isoforms are targeted to strikingly distinct and independent sites in the cell, permitting unique and independent roles for each of the isoforms in regulating discrete cellular processes.
Reversible phosphorylation of protein substrates plays an essential role in general metabolic regulation. The overall state of phosphorylation of substrates regulates such fundamental processes as gene expression, cell cycle progression, and maintenance of the differentiated state. The state of phosphorylation of specific substrates is, in turn, maintained by a highly regulated balance between specific protein kinases and protein phosphatases. In addition to the complex controls that regulate the state of activation of different kinases and phosphatases, the phosphorylation status of substrates can also be controlled by selective targeting of kinases and phosphatases to subcellular loci (Hubbard and Cohen, 1993; Faux and Scott, 1996).
The problem of targeting is particularly important for phosphatases. Human cells are estimated to have as many as 2,000 protein kinases (Hunter, 1995). Thus, a large number of serine/threonine kinases are capable of controlling a variety of metabolic events through substrate specificity, which is sometimes exquisitely selective. In contrast, there are relatively few families of serine/threonine phosphatases, and therefore they each must have a much broader range of targets. The fact that relatively few serine/threonine protein phosphatases are known raises the intriguing possibility that a small number of protein phosphatases might specifically regulate a large number of phosphorylation events and cellular processes by being targeted to various subcellular loci (Faux and Scott, 1996).
Protein phosphatase-1 (PP-1),1 an important family of serine/threonine phosphatases, is conserved in sequence among eukaryotes and regulates numerous independent processes in mammalian cells (Cohen, 1989; Shenolikar, 1994). In fibroblasts, PP-1 is required for spliceosome assembly (Mermoud et al., 1994; Misteli and Spector, 1996), for dephosphorylation of histone H1 (Paulson et al., 1996), for maintenance of the tumor suppressor pRb in an active state (Alberts et al., 1993; Ludlow et al., 1993), and for anaphase progression and exit from mitosis (Fernandez et al., 1992).
The PP-1 family has three major 37-kD catalytic subunit isoforms in mammalian cells (Sasaki et al., 1990; Barker et al., 1994). These isoforms exhibit 90% or greater identity in overall amino acid composition. It has recently been found that the different isoforms can all be expressed in the same cell (Puntoni and Villa-Moruzzi, 1997). Regulatory subunits that target PP-1 to myosin or glycogen in muscle and to nuclei in fibroblasts have been identified (Tang et al., 1991; Shimizu et al., 1994; Faux and Scott, 1996). It is possible that the different isoforms may be targeted individually by association with unique regulatory subunits.
The different PP-1 isoforms contain a strong sequence divergence in their COOH-terminal 30 amino acids, and this has allowed the production of isoform-specific antibodies (da Cruz e Silva et al., 1995; Villa-Moruzzi et al., 1996). Here we have used isoform-specific antibodies to perform immunofluorescent localization studies in mammalian cells in culture, to determine if individual isoforms show evidence of independent targeting.
We find that PP-1 α, γ1, and δ localize to distinct subcellular compartments during both interphase and mitosis. All PP-1 isoforms are present in nuclei, as well as in the cytoplasm, during interphase. Within the nucleus, PP-1 α associates with the nuclear matrix, whereas PP-1 γ1 localizes to the nucleolus, and PP-1 δ is associated with whole chromatin. During mitosis, the PP-1 isoforms also localize differentially. PP-1 α localizes to centrosomes, while PP-1 γ1 is associated with microtubules of the mitotic spindle. In contrast, we find that PP-1 δ is strongly localized to chromosomes. Our results thus present the possibility that each of the PP-1 isoforms is independently regulated and has distinct cell targets and roles in cellular regulation.
Materials and Methods
Cell Culture
HeLa cells were grown as monolayers in DME (GIBCO BRL, Paisley, UK). Manca (human non-Hodgkin's lymphoma) cells (Nishikori et al., 1984) were grown in suspension in RPMI 1640 medium (GIBCO BRL). HeLa and Manca cell cultures were supplemented with 5% bovine calf serum (Hyclone Labs, Logan, UT). All cells were maintained in a humid incubator at 5% CO2 and 37°C.
Antibodies
Peptide affinity-purified rabbit isoform-specific antibody to PP-1 α (RU34) (da Cruz e Silva et al., 1995) was a generous gift from Drs. Edgar da Cruz e Silva and Paul Greengard (Rockefeller University, New York). Peptide affinity-purified rabbit isoform-specific antibodies to PP-1 γ1 and PP-1 δ have been previously described (Villa-Moruzzi et al., 1996). Anti– β-tubulin ascites antibody (TUB 2.1) was from Sigma Chemical Co. (St. Louis, MO). Human autoimmune serum B.S., which recognizes centromere protein-A (CENP-A), has been described previously (Palmer et al., 1987). Secondary antibodies included FITC-conjugated affinity-purified goat anti–rabbit IgG antibodies from Cappel Laboratories (West Chester, PA) and cyanine-3–conjugated goat anti–mouse IgG antibodies from Jackson ImmunoResearch Laboratories (West Grove, PA).
Immunofluorescence Microscopy
HeLa cells were grown on poly-lysine–coated coverslips for a minimum of 24 h before fixation. Cells were fixed with 1% paraformaldehyde-PBS for 2 min, followed by −20°C methanol for 10 min and treatment with 0.5% NP-40 in PBS for 2 min. Fixation with 2% paraformaldehyde alone gave similar results. Washes with PBS, incubation with primary and secondary antibodies, and counterstaining with propidium iodide were as described previously (Andreassen and Margolis, 1994).
Images were collected with a MRC-600 Laser Scanning Confocal Apparatus (BioRad Microscience Division, Herts, UK) coupled to a Nikon Optiphot microscope (Melville, NY). Composite whole cell images were generated from serial optical sections representing the entire depth of field using Comos software (BioRad Microscience Division).
Nuclear Extraction for Microscopy
For immunofluorescent localization of PP-1 isoforms after nuclear extraction, HeLa cells were grown on poly-lysine–coated coverslips for a minimum of 48 h and then subjected to permeabilization and cell extraction. For extraction, cells were lysed with 0.5% Triton X-100 in 10 mM Pipes, pH 7.0, 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, and 1 mM EGTA containing 1 mM PMSF for 2 min (Zeng et al., 1994). After permeabilization, RNA and DNA were digested with 100 μg/ml RNase A and 100 μg/ml DNase I (Sigma Chemical Co.), respectively, for 20 min in the buffer described above. Digested chromatin was extracted 5 min with 250 mM ammonium sulfate in the same buffer after digestion of DNA. Permeabilization, digestion, and extraction were all performed at ambient temperature. After each step, cells were fixed for immunofluorescence microscopy as described above.
Preparation of Cellular Fractions for Immunoblotting
Nuclear Isolation.
To determine the presence of each PP-1 isoform in both nuclear and cytoplasmic fractions, Manca cells were fractionated by a modification of the procedure described by Palmer et al. (1987) and immunoblotted. Exponentially growing Manca cells were collected by centrifugation and washed with 3.75 mM Tris-HCl, pH 7.4, 15 mM KCl, 3.75 mM NaCl, 125 μM spermidine, 37.5 μM spermine, 250 μM EDTA, and 50 μM EGTA with 30% (vol/vol) glycerol, 15 mM β-mercaptoethanol, 10 μM aprotinin, 10 μM leupeptin, and 100 μM PMSF. Cells were then resuspended in 5 ml of the same buffer containing 0.2% Triton X-100 and incubated at 4°C for 30 min. Cells were homogenized by 20 strokes of a Dounce-A pestle, after which nuclei were determined to be free of cytoplasm by phase-contrast microscopy. Nuclei were collected by centrifugation (1,000 g, 10 min), and the nuclear and cytoplasmic fractions were adjusted to equivalent volumes with sample buffer for SDS-PAGE.
Mitotic Spindle.
The association of PP-1 isoforms with the mitotic spindle was determined by comparing the residual fractions in which microtubules were either stabilized with taxol (Schiff and Horwitz, 1980) or depolymerized with nocodazole (Jordan et al., 1992) after permeabilization in a microtubule-stabilizing buffer (Gorbsky and Ricketts, 1993). Mitotic cells were selectively detached after treatment with either taxol (5 μg/ml) or nocodazole (1 μg/ml) for 16 h. Mitotic indices of detached cells were greater than 90%. After collection, cells were permeabilized 2 min at 37°C in 60 mM Pipes, 25 mM Hepes, 10 mM EGTA, and 2 mM MgCl2, pH 6.9 (PHEM; Gorbsky and Ricketts, 1993) containing 0.2% Triton X-100, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 0.1 mM PMSF. Residual pellets were then collected at 37°C by centrifugation (300 g, 2 min), and fractions were prepared in sample buffer for SDS-PAGE.
Chromosomes.
The association of each PP-1 isoform with chromosomes was determined by its release into the soluble fraction after nuclease digestion of a chromosome fraction. Residual cell pellets containing chromosomes were prepared by lysis of selectively detached mitotic cells after treatment with 1.0 μg/ml nocodazole. Cells were permeabilized in PHEM containing 0.1% NP-40, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 0.1 mM PMSF. After 2 min, cells were collected by centrifugation and digested 30 min at 37°C with 40 μg/ml DNAse I. Soluble and residual fractions were then separated by centrifugation (300 g, 2 min) and analyzed as above.
Immunoblotting
Interphase HeLa cells were collected by trypsinization and mitotic cells by selective detachment after arrest with 0.04 μg/ml nocodazole. Cells were then lysed in 50 mM Tris-HCl, pH 7.4, 250 mM NaCl, 5 mM EGTA, 0.1% NP-40, 10 μg/ml aprotonin, 10 μg/ml leupeptin, and 1.0 mM PMSF for 30 min on ice. Lysates were resolved on 12% polyacrylamide gels, and gel-separated proteins were then transferred to nitrocellulose sheets using a semidry blotting apparatus, blocked with 5% nonfat milk, incubated overnight with primary antibodies, washed, and then incubated with HRP-conjugated goat anti–rabbit IgG secondary antibodies, as previously described (Andreassen and Margolis, 1994). Protein–antibody complex was detected by enhanced chemiluminescence (Amersham Corp., Arlington Heights, IL).
Isoform specificity of each antibody was tested by immunoblotting against equivalent amounts of each recombinant PP-1 isoform (Zhang et al., 1993; protein kindly provided by Dr. E.Y.C. Lee, New York Medical College, Valhalla, NY).
Results
Specificity of PP-1 Isoform Antibodies
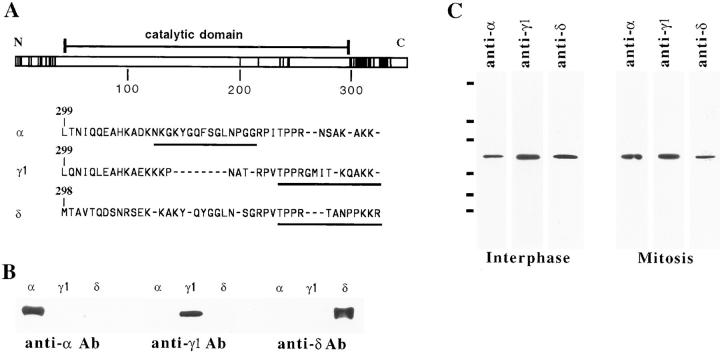
The human α, γ1, and δ isoforms of PP-1 have 90% or greater identity in amino acid sequence (Barker et al., 1994). The catalytic domains (amino acids 42–298 of PP-1 α) (Peruski et al., 1993) have greater than 97% homology between isoforms and have similar activities in vitro (Zhang et al., 1993). A small number of amino acid substitutions are conserved among mammalian species in the first 35 amino acids of sequence, upstream of the catalytic domain (Fig. 1 A). However, by far the greatest sequence variance occurs in the COOH-terminal 25–33 amino acids. The COOH-terminal divergence in sequence between isoforms may be of significance, as the COOH-terminal isoform-specific sequences are very highly conserved amongst mammalian species (Sasaki et al., 1990; Barker et al., 1994).
Figure 1.
Isoforms of PP-1 are most divergent at their COOH termini. (A) The sequences of human PP-1 α, γ1, and δ have been aligned and compared in this schematic representation. Each amino acid difference between any pair of PP-1 isoforms (Barker et al., 1994) is indicated by a solid vertical line. There is a modest amount of divergence at the NH2 terminus, but most divergence occurs proximal to the COOH terminus. There are few differences within the conserved catalytic domain (amino acids 42–98 in human PP-1 α; Peruski et al., 1993), indicated by a horizontal bar above the diagram. Single letter code sequences of PP-1 α, γ1, and δ at their divergent COOH termini are shown below the diagram. The peptide sequences used for the generation of isoform-specific antibodies are underlined. (B) Cross-blots of the PP-1 α, γ1, and δ antibodies against all three isoforms demonstrate that each antibody is isoform specific. (C) Antibodies generated against COOH-terminal peptides from PP-1 α, γ1, and δ each specifically recognize a 37-kD protein corresponding to PP-1 in blots of lysates from either interphase or mitotic HeLa cells. Bars at the left margin indicate size markers: 105, 82, 45, 33, 29, and 19 kD, respectively.
The presence of COOH-terminal isoform-specific sequence has allowed the production of antibodies that specifically recognize each of the isoforms in mammalian cells (da Cruz e Silva et al., 1995; Villa-Moruzzi et al., 1996). Each of the antibodies used in our study is specific for a single isoform. The specificity has been tested by cross-blotting procedures in which each antibody was used to probe each of the isoforms, expressed as recombinant proteins (Fig. 1 B; see also da Cruz e Silva et al., 1995; Villa-Moruzzi et al., 1996). Furthermore, each of the isoform-specific antibodies recognizes a single protein of 37 kD in both interphase and mitotic HeLa whole cell extracts (Fig. 1 C) and is therefore specific for PP-1. In accord with previous results (Puntoni and Villa-Moruzzi, 1997), the α, γ1, and δ isoforms of PP-1 are all expressed in HeLa cells.
Differential Localization of PP-1 Isoforms in HeLa Cells at Interphase and Mitosis
Although the PP-1 isoforms are highly homologous and have nearly identical catalytic domains, it is possible that either the conserved NH2-terminal or COOH-terminal sequence divergence might target the different isoforms to specific sites where they might have unique functions. We therefore tested for differences in localization of the antigens by immunofluorescence microscopy. In whole cell images generated from serial sections collected by confocal microscopy, we find that each isoform is evident both in the cytoplasm and nuclei of HeLa cells during interphase (Fig. 2). This is consistent with studies showing both nuclear and cytoplasmic PP-1 activity in interphase HeLa cells (Puntoni and Villa-Moruzzi, 1997). Controls treated without anti–PP-1 primary antibody do not display a detectable signal either in nuclei or the cytoplasm (data not shown).
Figure 2.
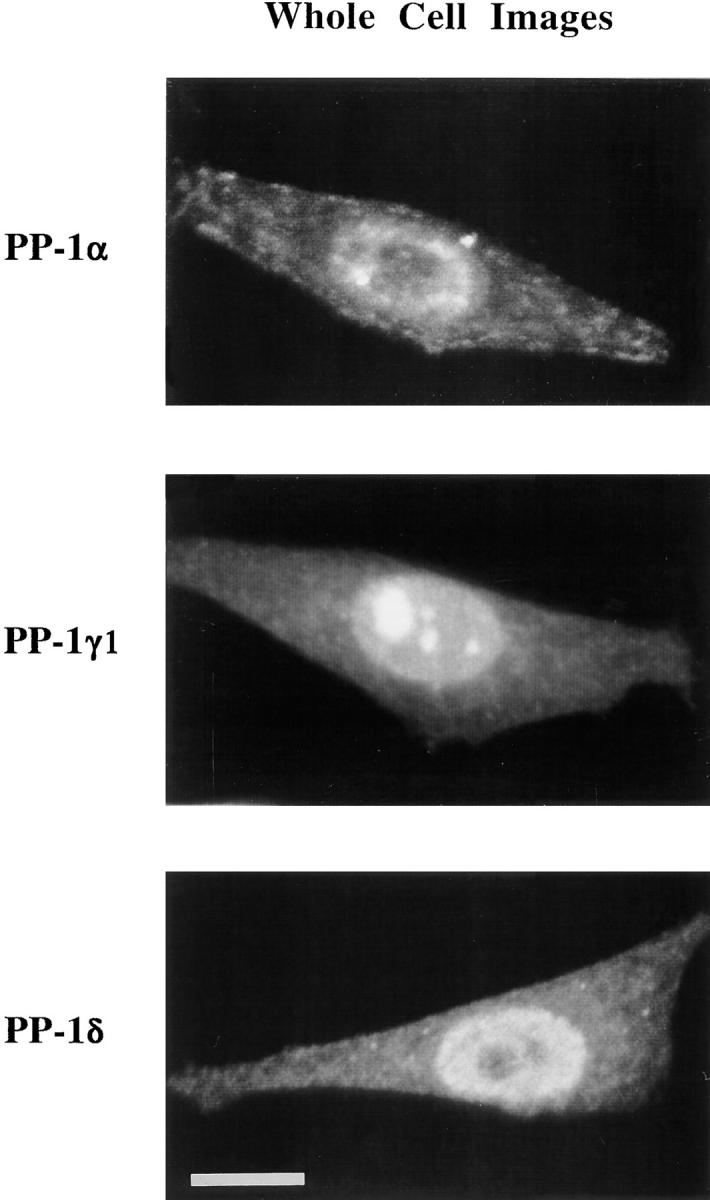
PP-1 α, γ1, and δ are each localized to both cytoplasm and nuclei in interphase HeLa cells. Images, representing whole cells, were generated from optical sections throughout the cell depth that were collected by confocal microscopy. A detailed comparison of isoform distributions in nuclei by optical sections of interphase cells is shown in Fig. 3 A. Bar, 10 μm.
We used optical sections obtained by confocal microscopy to examine in greater detail the specific localization of each PP-1 isoform both during interphase and mitosis in HeLa (Fig. 3 A). During interphase, we find that PP-1 α, γ1, and δ all are present in HeLa cell nuclei but that they localize to distinct nuclear compartments. Image overlay of PP-1 α against a propidium iodide counterstain shows that this isoform has a nonuniform distribution in nuclei (Fig. 3 A) and is excluded from nucleoli, which are discernible by strong propidium iodide staining. PP-1 α also concentrates on the centrosome, which is adjacent to the nucleus in interphase cells (Fig. 3 A). PP-1 δ is also excluded from nucleoli but displays a more homogeneous distribution elsewhere in the nucleus than PP-1 α. In contrast, PP-1 γ1 localizes preferentially to the nucleolus, yielding a yellow coloration where propidium iodide and PP-1 γ1 overlap (Fig. 3 A). A previous immunolocalization study had demonstrated that PP-1 localizes to nuclei in rat embryo fibroblasts, but it used antibodies that did not distinguish between PP-1 isoforms (Fernandez et al., 1992). Cytoplasmic signal is less concentrated than nuclear signal and is not uniformly detected in the optical sections shown in Fig. 3 A.
Figure 3.
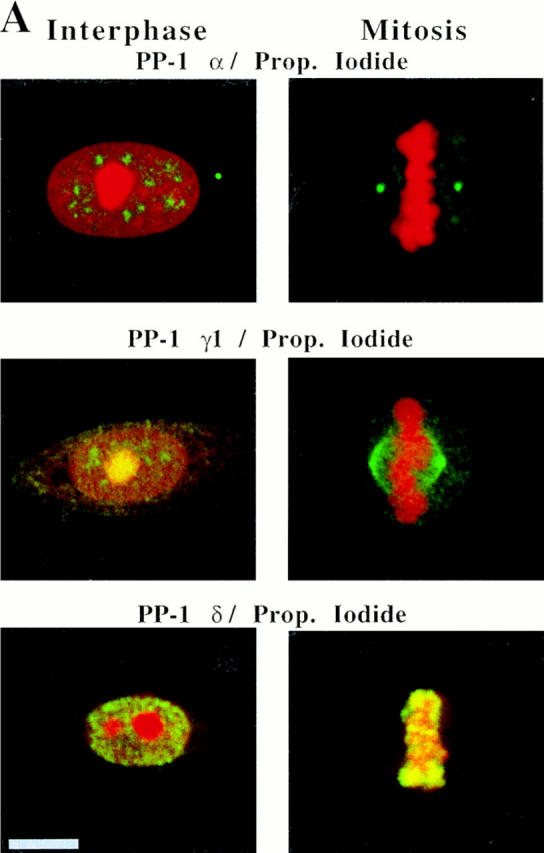
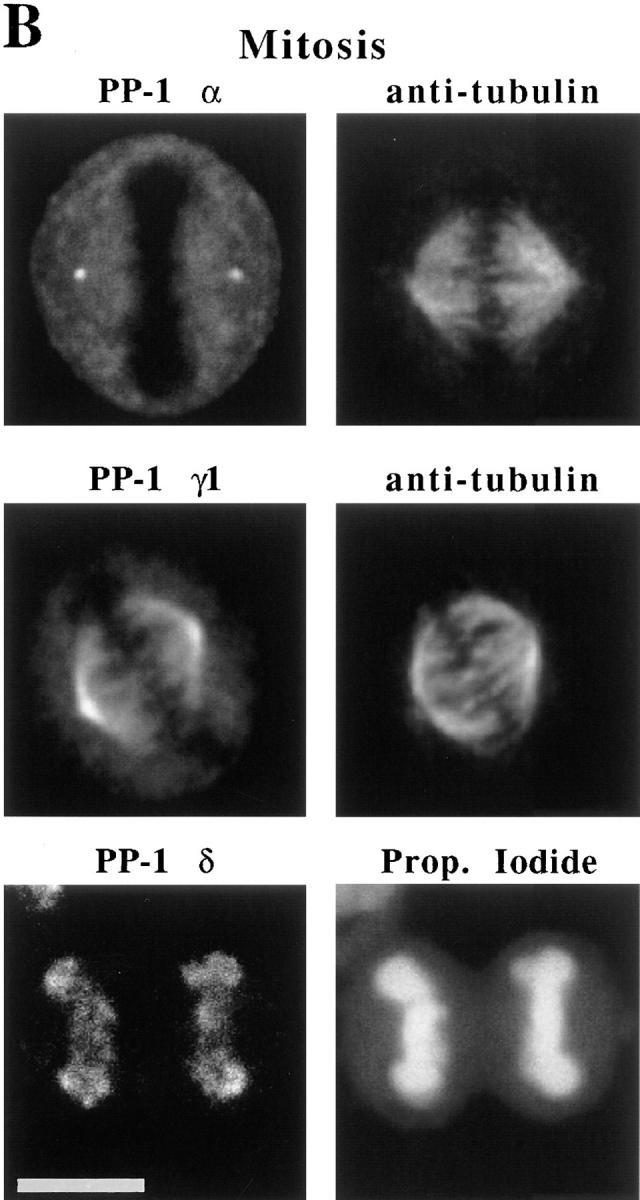
PP-1 isoforms are differentially localized both during interphase and mitosis in HeLa cells. (A) Images for PP-1 α, γ1, and δ (green) merged with respective images of propidium iodide counterstain (red) are shown for cells at interphase and at mitotic metaphase. All primary antibodies were detected with FITC-conjugated secondary antibodies. Each isoform is present in the nucleus at interphase. PP-1 γ1 localizes to the nucleolus, whereas PP-1 α and δ distribute elsewhere in the nucleus. At mitotic metaphase, PP-1 α, γ1, and δ antigens are distributed to the centrosome, mitotic microtubules, and chromosomes, respectively. Nucleoli and chromosomes are strongly stained by propidium iodide at interphase and mitosis, respectively. Images are yellow where the green and red signals overlap. A centrosome adjacent to the nucleus of an interphase cell is labeled with PP-1 α–specific antibody. (B) Separated images show PP-1 isoforms are specifically associated either with the mitotic spindle, centrosomes, or chromosomes of HeLa cells. (Top) Double-label immunofluorescence images of PP-1 α (left) and antitubulin (right) in a metaphase cell demonstrate that PP-1 α is concentrated at the centrosome. (Middle) Double-label immunofluorescence images of PP-1 γ1 (left) and antitubulin (right) show that PP-1 γ1 is localized to spindle microtubules at metaphase. (Bottom) PP-1 δ (left) remains associated with chromosomes throughout mitosis, as shown here for a telophase cell. The propidium iodide counterstain (right) confirms that the cell is in telophase. Bars, 10 μm.
We have determined that the distinctive nuclear distributions of the PP-1 isoforms observed in HeLa cells are also present in other mammalian cell lines. In addition to our study of PP-1 isoform distribution in HeLa, which are epitheloid carcinoma cells, we have also examined the nuclear localization of PP-1 isoforms in transformed human lymphocyte (Manca) cells (Nishikori et al., 1984) and in nontransformed CHO fibroblasts and rat embryonal fibroblasts (REF-52) (data not shown). Immunoblots demonstrate the same antibody specificity for PP-1 α, γ1, and δ in Manca cell extracts as in HeLa extracts (data not shown). Our results with these other cell lines show that interphase distributions of the isoforms are identical to those in HeLa cells.
We have also examined the localization of PP-1 α, γ1, and δ in HeLa cells during mitotic metaphase using optical sections (shown as two-color overlays in Fig. 3 A). PP-1 α shows a strong association with the centrosome but is excluded from chromosomes whose position is indicated by propidium iodide stain. PP-1 γ1 is present throughout the mitotic spindle but shows a higher concentration near the centrosomes (Fig. 3 A). Like PP-1 α, PP-1 γ1 is excluded from chromosomes (especially evident in separated channels; see Fig. 3 B). PP-1 δ, by contrast with the other two isoforms, is present predominantly on chromosomes and does not show any localization to the mitotic spindle.
We have examined the mitotic distributions of PP-1 isoforms in greater detail in Fig. 3 B. Here, double-label immunofluorescence microscopy for PP-1 α and antitubulin shows that PP-1 α is apparently centrosome associated (Fig. 3 B, top). Localization of PP-1 α to the spindle poles has been confirmed by digital overlay of the images for PP-1 α and tubulin (data not shown). By contrast, comparison of PP-1 γ1 and tubulin distribution demonstrates that PP-1 γ1 associates with microtubules of the mitotic spindle, but with an apparently higher concentration near the centrosomes (Fig. 3 B, middle). PP-1 γ1 remains associated with the spindle throughout mitosis, becoming concentrated near the spindle poles during telophase (data not shown). It is clear from the separated images that neither PP-1 α nor PP-1 γ1 associates with chromosomes. By contrast, PP-1 δ is present on metaphase chromosomes (Fig. 3 A), and it remains associated with chromosomes throughout mitosis, as is evident in a telophase image (Fig. 3 B, bottom). The differential associations of PP-1 α, γ1, and δ with the centrosome, mitotic spindle, and chromosomes, respectively, are not sensitive to detergent extraction (data not shown). The distribution of each isoform observed during mitosis is also conserved in Manca, CHO, and REF-52 cells (data not shown). We conclude that the immunofluorescence data demonstrate that the different PP-1 isoforms are targeted to markedly different sites in both interphase and mitotic cells. These data are summarized in Table I.
Table I.
PP-1 Isoform Localization
| PP-1 α | PP-1 γ 1 | PP-1 δ | ||||
|---|---|---|---|---|---|---|
| Interphase | nuclear matrix, centrosomes | nucleoli | chromatin | |||
| Mitosis | centrosomes | mitotic spindle | chromosomes |
The localization of each PP-1 isoform to nuclei was confirmed by immunoblots of nuclei isolated from Manca cells (Fig. 4). Manca cells, which are transformed human lymphocytes (Nishikori et al., 1984), were used for this procedure since their nuclei can be separated cleanly from cytoplasm. Each PP-1 isoform localizes to nuclei in Manca cells, with sublocalization similar to that of HeLa cells (data not shown). Effective separation of nuclei from cytoplasm has been confirmed by immunoblots for tubulin, which show that isolated nuclei are devoid of cytoskeletal and cytoplasmic contamination (Fig. 4). By contrast, the centromeric protein CENP-A (Palmer et al., 1987) is present in isolated nuclei but is absent from the cytoplasmic fraction, thus demonstrating that the cytoplasmic fraction is devoid of nuclear contamination. Each of the three PP-1 isoforms is present both in isolated nuclei and in the cytoplasmic fraction, and all are distributed roughly equivalently between the nucleus and cytoplasm.
Figure 4.
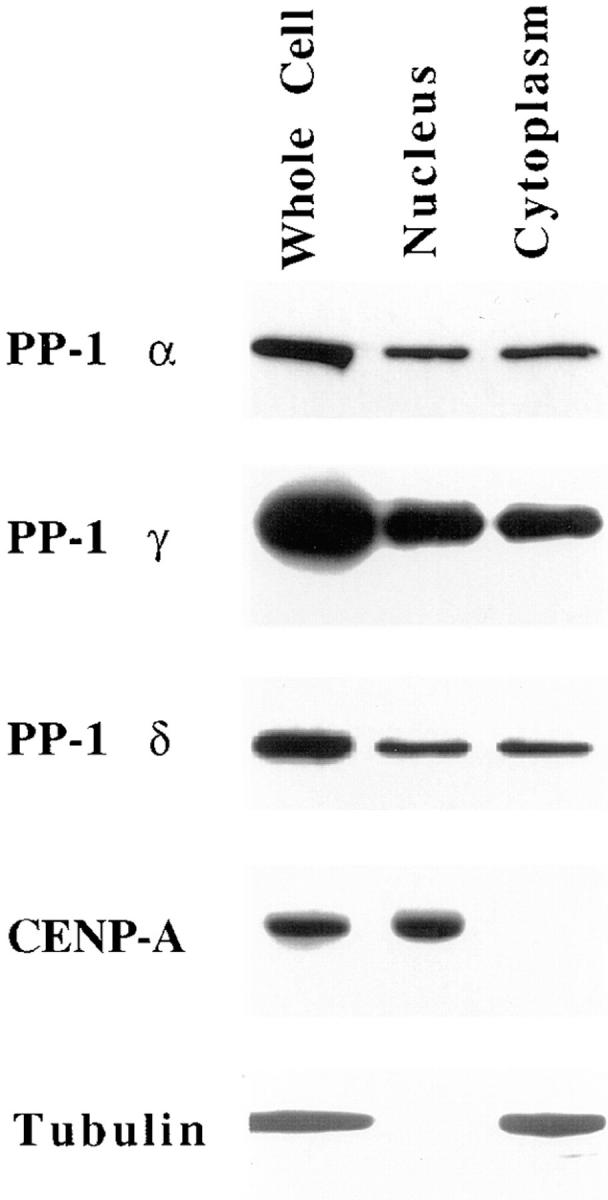
Each PP-1 isoform is present in isolated nuclei. Immunoblots of Manca whole cell lysates, of isolated nuclei, and of the released cytoplasmic fraction are shown. All lanes were loaded on the basis of cell equivalence. The presence of each PP-1 isoform both in nuclei and cytoplasm is demonstrated by immunoblots probed for PP-1 α, γ1, and δ, respectively. Samples were also exposed to antitubulin antibodies to show the purity of isolated nuclei, and to CENP-A antiserum to show the purity of the cytoplasmic fractions.
PP-1 has been implicated in the regulation of spliceosome activity, cAMP response element binding (CREB)-dependent transcription, and control of S phase progression (Hagiwara et al., 1992; Walker et al., 1992; Mermoud et al., 1994; Misteli and Spector, 1996). The differential distribution of the PP-1 isoforms in nuclei suggests that the different isoforms might independently control these distinct processes. To further analyze the distribution of the different PP-1's within distinct nuclear compartments, we performed indirect immunofluorescence microscopy after nuclear permeabilization and extraction of either RNA or chromatin (Fig. 5).
Figure 5.
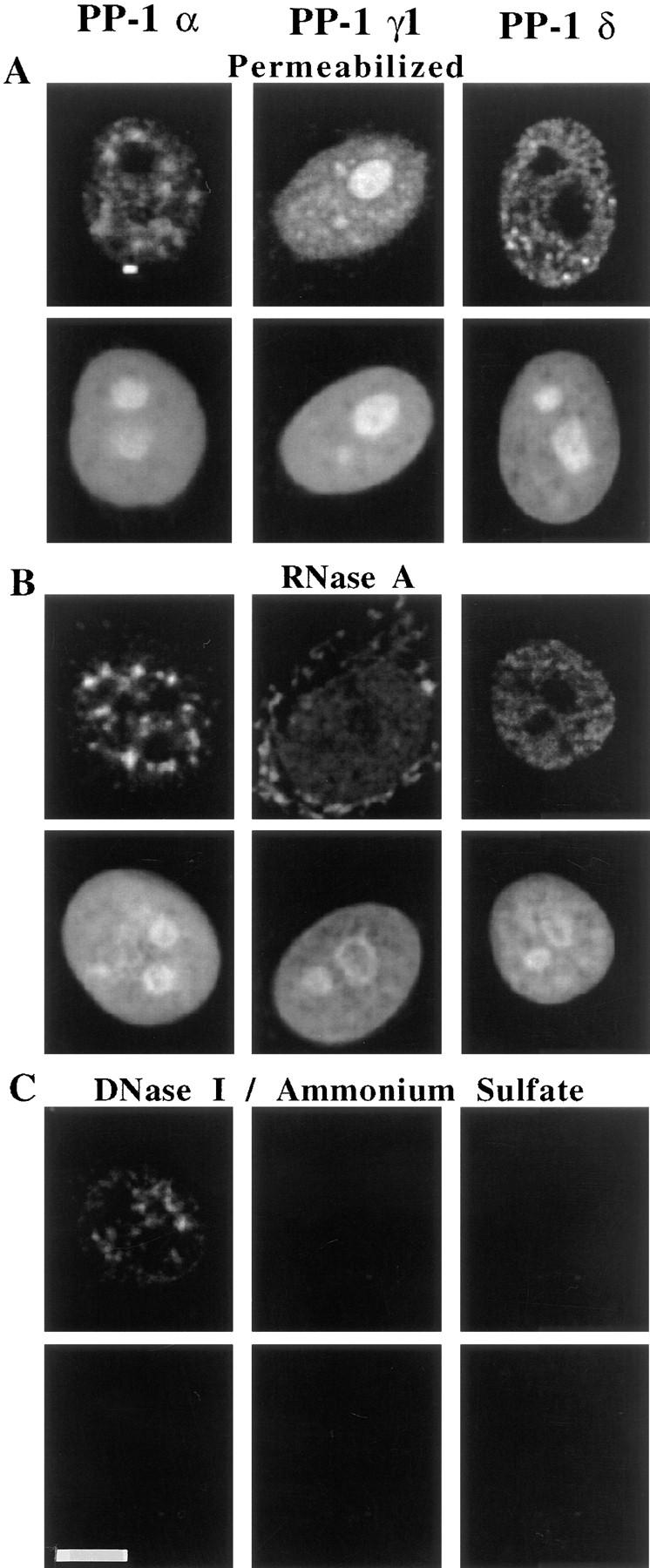
Nuclear extraction demonstrates that the different PP-1 isoforms localize to distinct nuclear compartments. HeLa cells were fixed and prepared for immunofluorescence microscopy either (A) after permeabilization with Triton X-100, (B) permeabilization followed by RNA digestion, or (C) permeabilization followed by chromatin extraction through treatment with DNase I and then 0.25 M ammonium sulfate. PP-1 α, γ1, and δ are all present in nuclei after permeabilization. PP-1 γ1 is sensitive to RNA extraction, PP-1 δ is extracted with chromatin, and PP-1 α resists extraction through association with the nuclear matrix. Propidium iodide counterstain reveals both the extraction of nucleolar RNA by RNase treatment and the extraction of chromatin by the combination of DNase I and salt. Bar, 10 μm.
After permeabilization (Fig. 5 A), PP-1 α and δ still show exclusion from nucleoli, while PP-1 γ1 is found enriched in the nucleolar compartment. Upon digestion with RNase A (Fig. 5 B), PP-1 γ1 is extracted from nucleoli, and the signal is also diminished elsewhere in nuclei. We note, however, that perinuclear cytoplasmic PP-1 γ1 signal is consistently augmented by RNase treatment. By contrast, PP-1 α and δ are not sensitive to RNase A digestion.
Bulk chromatin can be removed from nuclei by the combination of digestion with DNase I and salt extraction (Fig. 5 C) (Berezney and Coffey, 1977). Indirect immunofluorescence microscopy after extraction of chromatin reveals that the nuclear signal of PP-1 δ is completely extracted by this treatment. We conclude that PP-1 δ is chromatin associated. In addition to being sensitive to RNase treatment, PP-1 γ1 signal, including that in the nucleolus, is eliminated by treatment with DNase I and ammonium sulphate. By contrast, the nuclear signal of nonnucleolar PP-1 α remains after extraction of either RNA or bulk chromatin. We conclude, therefore, that a subset of PP-1 α is associated with the nuclear matrix (Berezney and Coffey, 1977). These results demonstrate that the PP-1 α, γ1, and δ isoforms localize not only to different sites but also to different molecular compartments within HeLa nuclei.
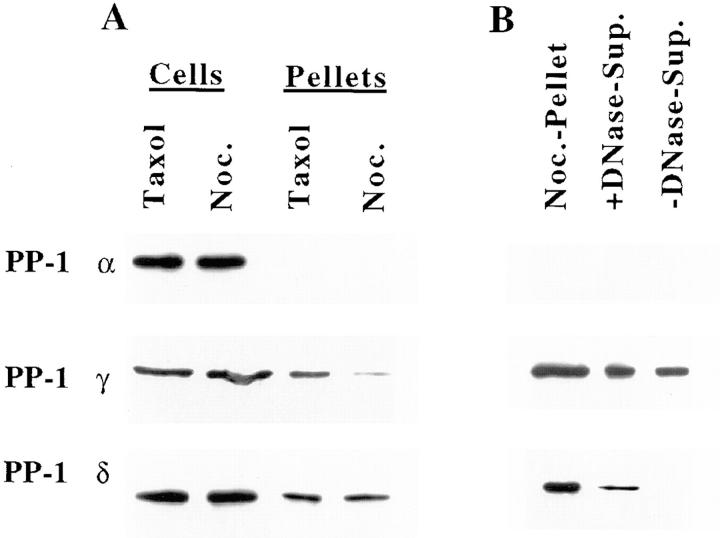
Fractions from HeLa cells were immunoblotted to confirm the differential localization of the PP-1 isoforms during mitosis (Fig. 6). To determine association with the mitotic spindle, cells were treated with either taxol, which stabilizes microtubule assembly (Schiff and Horwitz, 1980), or nocodazole, which induces depolymerization of microtubules (Jordan et al., 1992). After drug treatment, mitotic cells were selectively detached and were permeabilized with a microtubule-stabilizing buffer (Gorbsky and Ricketts, 1993). Western blots show PP-1 γ1 is associated with the microtubule-containing cell pellet from taxol-treated cells, but it is much diminished in pellets from nocodazole-treated cells that are devoid of microtubules (Fig. 6 A). By contrast, PP-1 δ is equally abundant in the cell pellets of both taxol- and nocodazole-treated cells. PP-1 α is not detectable in cell pellets from cells treated with either taxol or nocodazole (Fig. 6 A). We conclude that each PP-1 isoform is distributed distinctly in mitotic cells and that the distribution of PP-1 γ1, but not of PP-1 α or δ, is uniquely microtubule dependent.
Figure 6.
PP-1 isoforms distribute to different compartments in mitotic HeLa cells as assayed in immunoblots. PP-1 γ1, but not PP-1 α or δ, is enriched in microtubule fractions after cell lysis (A). Residual pellets from cells treated with taxol contain assembled microtubules, but pellets from cells treated with 1.0 μg/ml nocodazole (Noc.) are devoid of microtubules. PP-1 γ1 is preferentially associated with the residual cell pellet that contains microtubules after treatment with taxol. Whole cell lysates and residual cell pellets from cells treated with either taxol or nocodazole and loaded on the basis of equal cell number are shown. PP-1 δ is associated with chromosomes, as determined by release from the residual cell pellet after digestion with DNase I (B). Mitotic cells were collected by selective detachment, permeabilized, and incubated in digestion buffer either with (+) or without (−) DNase I, and supernatants (Sup.) were loaded onto gels. The residual cell pellet after lysis, loaded on the basis of equal cell number, is shown for reference. The majority of PP-1 δ is present in the cell supernatant from nocodazole-treated cells (see Fig. 6 A). Therefore, the blot of PP-1 δ shown here was exposed extensively to bring out the pellet fraction to examine its extractability by digestion with DNase I.
After lysis of cells arrested in mitosis (Fig. 6, A and B), both PP-1 γ1 and δ are present in the cell pellet, which contains both cytoskeleton and chromosomes, as determined by immunofluorescence microscopy and immunoblotting (data not shown). The association of PP-1 δ with chromosomes has been confirmed by its solubilization after digestion of lysed cells with DNase I (Fig. 6 B). The release of PP-1 δ into the supernatant is specific to DNase treatment and does not occur in a mock-digestion without DNase I. PP-1 α is absent from the cell pellet after permeabilization, and PP-1 γ1, which is weakly present in the pellet fraction (Fig. 6 A), is not preferentially solubilized by digestion with DNase I (Fig. 6 B). The evidence thus supports the unique association of PP-1 δ, in part, with chromosomes.
Discussion
PP-1 has many important regulatory roles in cell metabolism (Cohen, 1989; Shenolikar, 1994). Although the PP-1 family is composed of several highly conserved catalytic isoforms, it has not been evident that these isoforms might have distinct functions within a single cell. Here we report that the different isoforms of PP-1 are all present within each of several different cell types from different species. Furthermore, the PP-1 isoforms each localize to distinct sites both in mitotic cells and in interphase nuclei. A previous study of the subcellular localization of PP-1 used an antibody raised against the common catalytic domain sequence and thus did not distinguish between PP-1 isoforms (Fernandez et al., 1992). In contrast, using isoform-specific antibodies raised against distinct COOH-terminal sequences, we have found an intricate differential distribution pattern of PP-1 isoforms among different cellular organelles.
PP-1 can be targeted and regulated by association with targeting subunits (Hubbard and Cohen, 1993; Stuart et al., 1994; Faux and Scott, 1996). Our finding of independent localization of PP-1 isoforms suggests that each catalytic isoform may be differentially localized by association with one or more unique targeting subunits. Assuming this is true, many PP-1–targeting subunits remain to be identified. At each specific site, PP-1 is likely to have unique substrates. Because the targeting subunits are themselves subject to regulation, for example by phosphorylation (MacKintosh et al., 1988; Beullens et al., 1993), independent targeting and regulation of PP-1 isoforms could permit PP-1 to specifically and independently control multiple cellular processes. Finally, given the demonstrated role of PP-1 in the regulation of mitosis (Doonan and Morris, 1989; Ohkura et al., 1989; Axton et al., 1990; Fernandez et al., 1992) and the distinct localizations we have found, our results suggest a specific and independent role for each PP-1 isoform in mitosis.
Isoforms of the PP-1 Catalytic Subunit Are Differentially Targeted
We have found that PP-1 α, γ1, and δ show different subcellular localization both during interphase and mitosis (Table I). During interphase, each of the isoforms is concentrated in distinct nuclear compartments. For example, PP-1 γ1 is concentrated at nucleoli, while PP-1 α and δ appear to be excluded from nucleoli. Within this compartment, PP-1 α is partly associated with the nuclear matrix, while PP-1 δ is associated with the DNase-extractable chromatin fraction. During mitosis, PP-1 δ is associated with chromosomes. By contrast, PP-1 γ1 is associated with microtubules of the mitotic spindle, while PP-1 α is associated with the centrosome. These results suggest that PP-1 isoforms achieve specificity by targeting to different sites within the cell. Furthermore, these results strongly suggest that there is an isoform-specific regulation of various processes that have thus far been attributed to PP-1 activity without distinction of the isoform involved.
The catalytic subunits of PP-1 are localized by targeting subunits (Hubbard and Cohen, 1993; Stuart et al., 1994; Faux and Scott, 1996). In mammalia, subunits that target PP-1 to glycogen, myofibrils, and nuclei have been identified (Tang et al., 1991; Shimizu et al., 1994; Faux and Scott, 1996). It is clear that different isoforms can share association with certain targeting subunits (Alessi et al., 1993). However, our results suggest it is equally probable that specific catalytic subunits can be directed to different targets by association with unique targeting subunits. The subunits that might target PP-1 catalytic isoforms to distinct sites within nuclei and that might direct PP-1 δ to chromosomes, PP-1 α to centrosomes, and PP-1 γ1 to the mitotic spindle are presently unknown.
Because PP-1–targeting subunits can regulate substrate specificity and sensitivity to inhibitory proteins (Hubbard and Cohen, 1993), the function of PP-1 must be considered in the context of the specific catalytic–regulatory subunit complex. The targeting subunit itself can be regulated by phosphorylation (MacKintosh et al., 1988; Beullens et al., 1993), suggesting that PP-1 isoforms localized at unique sites can be independently regulated. Each isoform of the catalytic subunit of PP-1 could possibly associate with multiple targeting subunits, yielding further specificity of PP-1 targeting and regulation.
The specific localization of PP-1 isoforms that we have observed implicates a specific PP-1 isoform in the regulation of several site-specific processes previously ascribed generically to PP-1. For example, CREB-dependent phosphorylation, which regulates CREB-dependent transcription, is in turn regulated by PP-1 (Hagiwara et al., 1992). Given the association of PP-1 δ with chromatin, we suggest that this isoform may specifically regulate transcription. Other nuclear processes might also be regulated by site-specific activity of PP-1 isoforms. For example, PP-1 is required for spliceosome assembly (Mermoud et al., 1994; Misteli and Spector, 1996). Splicing factors display a punctate distribution and are associated with the nuclear matrix (Bisotto et al., 1995; Misteli and Spector, 1996). These are also characteristics of PP-1 α distribution, and they suggest that PP-1 α might uniquely play a role in pre-mRNA splicing. Similarly, the predominant localization of PP-1 γ1 to nucleoli suggests it might have a specific function in ribosome processing (Beullens et al., 1996).
During mitosis, a PP-1 activity has recently been found to be associated with chromosomes and to be involved in the dephosphorylation of histone H1 (Paulson et al., 1996). Since PP-1 δ, and not PP-1 α or PP-1 γ1, is associated with chromosomes, we suggest that this isoform may be the protein phosphatase responsible for the dephosphorylation of histone H1 and the regulation of decondensation of chromosomes at the end of mitosis. If true, it would be expected that PP-1 δ would remain associated with chromosomes through the end of mitosis when chromosome decondensation occurs, as we have observed (Fig. 3 B).
A role for PP-1 in the regulation of chromosome segregation and mitotic exit has been demonstrated previously (Doonan and Morris, 1989; Ohkura et al., 1989; Axton et al., 1990; Fernandez et al., 1992). In Drosophila, mutation of a single isoform of PP-1 causes mitotic arrest. By localizing to spindle microtubules, PP-1 γ1 might control chromosome segregation by regulating microtubule dynamics. Such localization is interesting in light of a recent report of a specific role for PP-1 in the control of microtubule dynamics during exit from mitosis in Xenopus extracts (Tournebize et al., 1997).
Alternatively, either PP-1 α or PP-1 γ1 might play a role at the metaphase–anaphase transition. Either of these PP-1 isoforms could be involved in checkpoint mechanisms that monitor mitotic spindle function and delay the onset of anaphase. Microinjection of mammalian fibroblasts with non–isoform-specific PP-1 antibodies induces arrest at metaphase (Fernandez et al., 1992). Also, the mitotic spindle is the site of degradation of cyclin B and of p34cdc2 kinase inactivation (Kubiak et al., 1993; Andreassen and Margolis, 1994; Tugendreich et al., 1995), both of which normally occur at the onset of anaphase (Pines and Hunter, 1991; Hunt et al., 1992). Since p34cdc2 can phosphorylate (Villa-Moruzzi, 1992) and inactivate PP-1 (Dohadwala et al., 1994; Puntoni and Villa-Moruzzi, 1997), degradation of cyclin B at the onset of anaphase might lead to the activation of PP-1 as a requirement for the onset or completion of anaphase (Kwon et al., 1997). Upon activation, either PP-1 γ1, which is associated with microtubules, or PP-1 α, which is associated with the centrosome, might regulate anaphase by local action at these sites.
The PP-1 α, γ1, and δ isoforms are products of distinct genes (Barker et al., 1993, 1994). Both PP-1 α and PP-1 γ1, but apparently not PP-1 δ, are expressed at elevated levels in certain human tumors (Sogawa et al., 1994a ,b). It is possible that these two isoforms have distinct roles in cell cycle regulation not shared by PP-1 δ. Our work now makes it important to pursue an understanding of the distinct site-specific roles that each of the PP-1 isoforms must play in regulation of the cell cycle, and possibly in tumorigenesis.
Acknowledgments
We are grateful to E.F. da Cruz e Silva and P. Greengard for providing affinity-purified anti–PP-1 α antibody, and Dr. E.Y.C. Lee for providing PP-1 recombinant proteins.
This work was supported in part by grants from the Association pour la Recherche sur le Cancer and from the International Human Frontiers of Sciences Program (R.L. Margolis) and the Associazione Italiana Ricerca sul Cancro (E. Villa-Moruzzi).
Abbreviations used in this paper
- CENP-A
centromere protein-A
- CREB
cAMP response element binding
- PP-1
protein phosphatase-1
Footnotes
Address all correspondence to Robert L. Margolis, Institut de Biologie Structurale Jean-Pierre Ebel (CEA-CNRS), 41 avenue des Martyrs, 38027 Grenoble cedex 1, France. Tel.: 33-4-76-88-96-16. Fax: 33-4-76-88-54-94.
References
- Alberts AS, Thorburn AM, Shenolikar S, Mumby MC, Feramisco JR. Regulation of cell cycle progression and nuclear affinity of the retinoblastoma protein by protein phosphatases. Proc Natl Acad Sci USA. 1993;90:388–392. doi: 10.1073/pnas.90.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Street AJ, Cohen P, Cohen PTW. Inhibitor-2 functions like a chaperone to fold three expressed isoforms of mammalian protein phosphatase-1 into a conformation with the specificity and regulatory properties of the native enzyme. Eur J Biochem. 1993;213:1055–1066. doi: 10.1111/j.1432-1033.1993.tb17853.x. [DOI] [PubMed] [Google Scholar]
- Andreassen PR, Margolis RL. Microtubule dependency of p34cdc2inactivation and mitotic exit in mammalian cells. J Cell Biol. 1994;127:789–802. doi: 10.1083/jcb.127.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axton JM, Dombradi V, Cohen PTW, Glover DM. One of the protein phosphatase 1 isoenzymes in Drosophila is essential for mitosis. Cell. 1990;63:33–46. doi: 10.1016/0092-8674(90)90286-n. [DOI] [PubMed] [Google Scholar]
- Barker HM, Craig SP, Spurr NK, Cohen PTW. Sequence of human protein serine/threonine phosphatase 1 γ and localization of the gene (PPP1CC) encoding it to chromosome bands 12q24.1-q24.2. Biochim Biophys Acta. 1993;1178:228–233. doi: 10.1016/0167-4889(93)90014-g. [DOI] [PubMed] [Google Scholar]
- Barker HM, Brewis ND, Street AJ, Spurr NK, Cohen PTW. Three genes for protein phosphatase 1 map to different human chromosomes: sequence expression and gene localisation of protein serine/threonine phosphatase 1 β (PPP1Cβ) Biochim Biophys Acta. 1994;1220:212–218. doi: 10.1016/0167-4889(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Berezney R, Coffey DS. Nuclear matrix: isolation and characterization of a framework structure from rat liver nuclei. J Cell Biol. 1977;73:616–637. doi: 10.1083/jcb.73.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beullens M, Van Eynde A, Bollen M, Stalmans W. Inactivation of nuclear inhibitory polypeptides of protein phosphatase-1 (NIPP-1) by protein kinase A. J Biol Chem. 1993;268:13172–13177. [PubMed] [Google Scholar]
- Beullens M, Stalmans W, Bollen M. Characterization of a ribosomal inhibitory polypeptide of protein phosphatase-1 from rat liver. Eur J Biochem. 1996;239:183–189. doi: 10.1111/j.1432-1033.1996.0183u.x. [DOI] [PubMed] [Google Scholar]
- Bisotto S, Lauriault P, Duval M, Vincent M. Colocalization of a high molecular mass phosphoprotein of the nuclear matrix (p255) with spliceosomes. J Cell Sci. 1995;108:1873–1882. doi: 10.1242/jcs.108.5.1873. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- da Cruz e Silva EF, Fox CA, Ouimet CC, Gustafson E, Watson SJ, Greengard P. Differential expression of protein phosphatase 1 isoforms in mammalian brain. J Neurosci. 1995;15:3375–3389. doi: 10.1523/JNEUROSCI.15-05-03375.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohadwala M, da Cruz e Silva EF, Hall FL, Williams RT, Carbonaro-Hall DA, Nairn AC, Greengard P, Berndt N. Phosphorylation and inactivation of protein phosphatase 1 by cyclin-dependent kinases. Proc Natl Acad Sci USA. 1994;91:6408–6412. doi: 10.1073/pnas.91.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan JH, Morris NR. The bimG gene of Aspergillus nidulans, required for completion of anaphase, encodes a homolog of mammalian phosphoprotein phosphatase 1. Cell. 1989;57:987–996. doi: 10.1016/0092-8674(89)90337-1. [DOI] [PubMed] [Google Scholar]
- Faux MC, Scott JD. More on target with protein phosphorylation: conferring specificity by location. Trends Biochem Sci. 1996;21:312–315. [PubMed] [Google Scholar]
- Fernandez A, Brautigan DL, Lamb NJC. Protein phosphatase type 1 in mammalian cell mitosis: chromosomal localization and involvement in mitotic exit. J Cell Biol. 1992;116:1421–1430. doi: 10.1083/jcb.116.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky GJ, Ricketts WA. Differential expression of a phosphoepitope at the kinetochores of moving chromosomes. J Cell Biol. 1993;122:1311–1321. doi: 10.1083/jcb.122.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M, Alberts A, Brindle P, Meinkoth J, Feramisco J, Deng T, Karin M, Shenolikar S, Montminy M. Transcriptional attenuation following cAMP induction requires PP-1 mediated dephosphorylation of CREB. Cell. 1992;70:105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- Hubbard MJ, Cohen P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem Sci. 1993;18:172–177. doi: 10.1016/0968-0004(93)90109-z. [DOI] [PubMed] [Google Scholar]
- Hunt T, Luca FC, Ruderman JV. The requirements for protein synthesis and degradation, and the control of destruction of cyclins A and B in the meiotic and mitotic cell cycles of the clam embryo. J Cell Biol. 1992;116:707–724. doi: 10.1083/jcb.116.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Thrower D, Wilson L. Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles: implications for the role of microtubule dynamics in mitosis. J Cell Sci. 1992;102:401–416. doi: 10.1242/jcs.102.3.401. [DOI] [PubMed] [Google Scholar]
- Kubiak JZ, Weber M, de Pennart H, Winston NJ, Maro B. The metaphase II arrest in mouse oocytes is controlled through microtubule- dependent destruction of cyclin B in the presence of CSF. EMBO (Eur Mol Biol Organ) J. 1993;12:3773–3778. doi: 10.1002/j.1460-2075.1993.tb06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y-G, Lee SY, Choi Y, Greengard P, Nairn AC. Cell cycle-dependent phosphorylation of mammalian protein phosphatase 1 by cdc2 kinase. Proc Natl Acad Sci USA. 1997;94:2168–2173. doi: 10.1073/pnas.94.6.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow JW, Glendening CL, Livingston DM, DeCaprio JA. Specific enzymatic dephosphorylation of the retinoblastoma protein. Mol Cell Biol. 1993;13:367–372. doi: 10.1128/mcb.13.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh C, Campbell DG, Hiraga A, Cohen P. Phosphorylation of the glycogen-binding subunit of protein phosphatase-1Gin response to adrenalin. FEBS Lett. 1988;234:189–194. doi: 10.1016/0014-5793(88)81331-0. [DOI] [PubMed] [Google Scholar]
- Mermoud JE, Cohen PTW, Lamond AI. Regulation of mammalian spliceosome assembly by a protein phosphorylation mechanism. EMBO (Eur Mol Biol Organ) J. 1994;13:5679–5688. doi: 10.1002/j.1460-2075.1994.tb06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli T, Spector DL. Serine/threonine phosphatase 1 modulates the subnuclear distribution of pre-mRNA splicing factors. Mol Biol Cell. 1996;7:1559–1572. doi: 10.1091/mbc.7.10.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikori M, Hansen H, Jhanwar S, Fried J, Sordillo P, Koziner B, Lloyd K, Clarkson B. Establishment of a near tetraploid B-cell lymphoma line with duplication of the 8;14 translocation. Cancer Genet Cytogenet. 1984;12:39–50. doi: 10.1016/0165-4608(84)90006-2. [DOI] [PubMed] [Google Scholar]
- Ohkura H, Kinoshita N, Miyatani S, Toda T, Yanagida M. The fission yeast dis2 +gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989;57:997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- Palmer DK, O'Day K, Wener MH, Andrews BS, Margolis RL. A 17-kD centromere protein (CENP-A) copurifies with nucleosome core particles and with histones. J Cell Biol. 1987;104:805–815. doi: 10.1083/jcb.104.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson JR, Patzlaff JS, Vallis AJ. Evidence that the endogenous histone H1 phosphatase in HeLa mitotic chromosomes is protein phosphatase 1, not protein phosphatase 2A. J Cell Sci. 1996;109:1437–1447. doi: 10.1242/jcs.109.6.1437. [DOI] [PubMed] [Google Scholar]
- Peruski LF, Jr, Wadzinski BE, Johnson GL. Analysis of the multiplicity, structure, and function of protein serine/threonine phosphatases. Adv Prot Phos. 1993;7:9–30. [Google Scholar]
- Pines J, Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle–dependent nuclear transport. J Cell Biol. 1991;115:1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntoni F, Villa-Moruzzi E. Protein phosphatase-1α, γ1, and δ: changes in phosphorylation and activity in mitotic HeLa cells and in cells released from the mitotic block. Arch Biochem Biophys. 1997;340:177–184. doi: 10.1006/abbi.1997.9889. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Shima H, Kitagawa Y, Irino S, Sugimura T, Nagao M. Identification of members of the protein phosphatase 1 gene family in the rat and enhanced expression of protein phosphatase 1α gene in rat hepatocellular carcinomas. Jpn J Cancer Res. 1990;81:1272–1280. doi: 10.1111/j.1349-7006.1990.tb02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenolikar S. Protein serine/threonine phosphatases—new avenues for cell regulation. Annu Rev Cell Biol. 1994;10:55–86. doi: 10.1146/annurev.cb.10.110194.000415. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Ito M, Miyahara M, Ichikawa K, Okubo S, Konishi T, Naka M, Tanaka T, Hirano K, Hartshorne DJ, Nakano T. Characterization of the myosin-binding subunit of smooth muscle myosin phosphatase. J Biol Chem. 1994;269:30407–30411. [PubMed] [Google Scholar]
- Sogawa K, Yamada T, Funamoto Y, Kohno K, Nishikawa H, Kishida F, Hamazaki F, Yamashita N, Matsumoto K. Selective increase in expression of isoform PP1 γ1 of type-1 protein phosphatase in chondrosarcoma cells. Res Commun Mol Pathol Pharm. 1994a;86:375–378. [PubMed] [Google Scholar]
- Sogawa K, Yamada T, Masaki T, Nishikawa H, Cai Y, Oka S, Norimatsu H, Matsumoto K. Enhanced expression of catalytic subunits of protein phosphatase type 1 and high S-phase fraction in liposarcoma. Res Commun Mol Path Pharm. 1994b;85:359–362. [PubMed] [Google Scholar]
- Stuart JS, Frederick DL, Varner CM, Tatchell K. The mutant type 1 protein phosphatase encoded by glc7-1 from Saccharomyces cerevisiae fails to interact productively with the GAC1-encoded regulatory subunit. Mol Cell Biol. 1994;14:896–905. doi: 10.1128/mcb.14.2.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang PM, Bondor JA, Swiderek KM, DePaoli-Roach AA. Molecular cloning and expression of the regulatory (RG1) subunit of the glycogen-associated protein phosphatase. J Biol Chem. 1991;266:15782–15789. [PubMed] [Google Scholar]
- Tournebize R, Andersen SSL, Verde F, Dorée M, Karsenti E, Hyman AA. Distinct roles of PP1 and PP-2A-like phosphatases in control of microtubule dynamics during mitosis. EMBO (Eur Mol Biol Org) J. 1997;16:5537–5549. doi: 10.1093/emboj/16.18.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugendreich S, Tomkiel J, Earnshaw W, Hieter P. CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell. 1995;81:261–268. doi: 10.1016/0092-8674(95)90336-4. [DOI] [PubMed] [Google Scholar]
- Villa-Moruzzi E. Activation of type-1 protein phosphatase by cdc2 kinase. FEBS Lett. 1992;304:211–215. doi: 10.1016/0014-5793(92)80621-m. [DOI] [PubMed] [Google Scholar]
- Villa-Moruzzi E, Puntoni F, Marin O. Activation of protein phosphatase-1 isoforms and glycogen synthase kinase-3β in muscle from mdxmice. Int J Biochem Cell Biol. 1996;28:13–22. doi: 10.1016/1357-2725(95)00119-0. [DOI] [PubMed] [Google Scholar]
- Walker DH, DePaoli-Roach AA, Maller JL. Multiple roles for protein phosphatase 1 in regulating the Xenopusearly embryonic cell cycle. Mol Biol Cell. 1992;3:687–698. doi: 10.1091/mbc.3.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, He D, Brinkley BR. Localization of NuMA protein isoforms in the nuclear matrix of mammalian cells. Cell Motil Cytoskel. 1994;29:167–176. doi: 10.1002/cm.970290208. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Bai G, Shima M, Zhao S, Nagao M, Lee EYC. Expression and characterization of rat protein phosphatases -1α, -1γ1, -1γ2, and -1δ. Arch Biochem Biophys. 1993;303:402–406. doi: 10.1006/abbi.1993.1301. [DOI] [PubMed] [Google Scholar]