Abstract
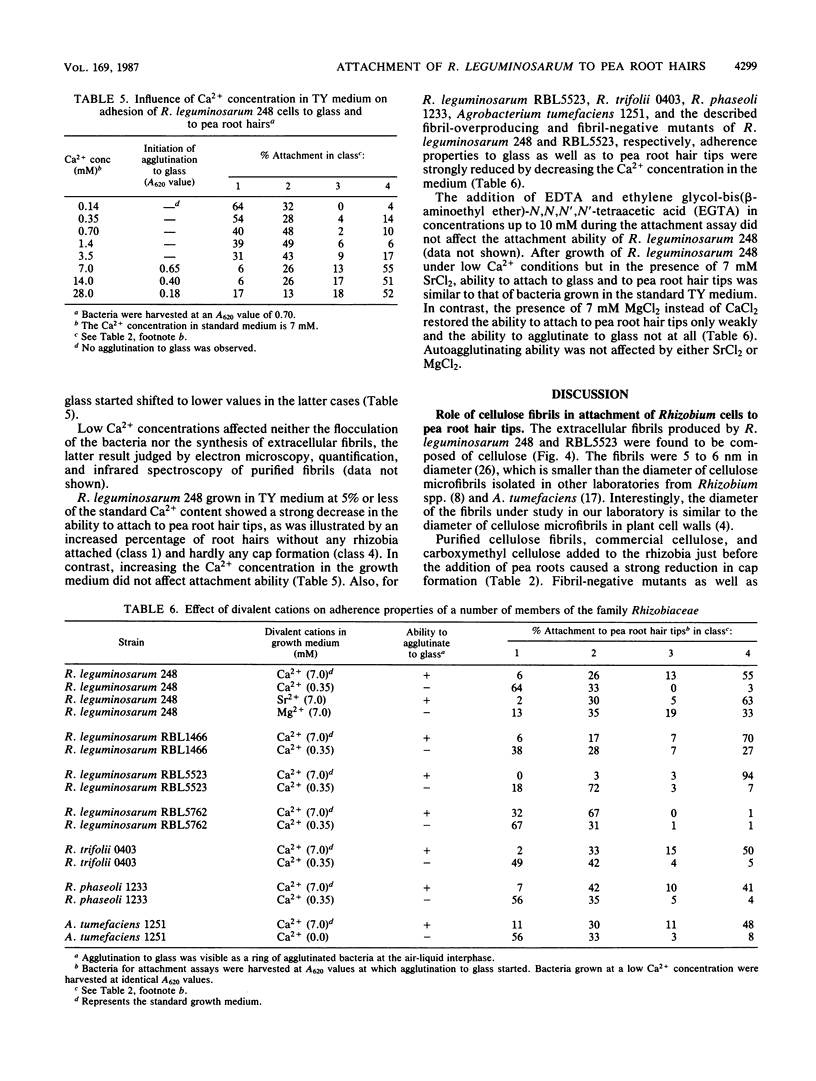
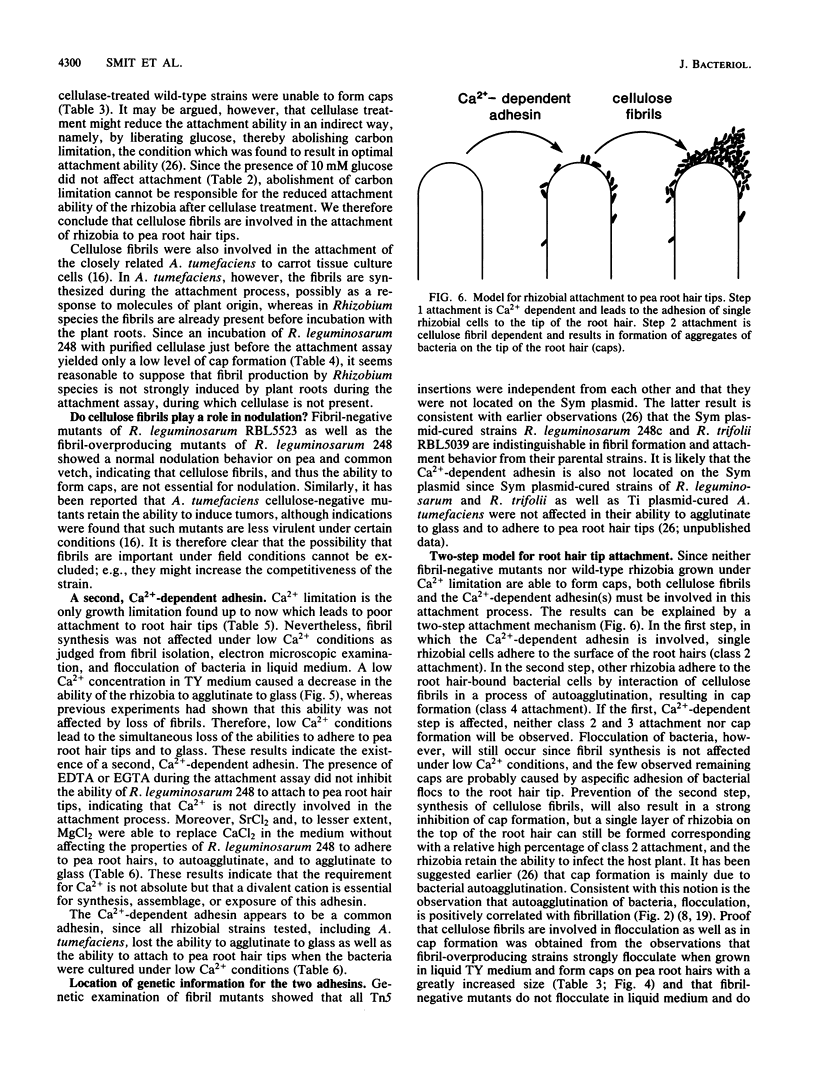
We have previously described an assay for the attachment of Rhizobium bacteria to pea root hair tips (cap formation) which was used as a model to study the attachment step in the nodulation process. Under all conditions tested, a positive correlation was observed between the percentage of fibrillated cells and the ability of these bacteria to form caps and to adhere to glass, suggesting that fibrils play a role in the attachment of Rhizobium leguminosarum to pea root hair tips and to glass (G. Smit, J. W. Kijne, and B. J. J. Lugtenberg, J. Bacteriol. 168:821-827, 1986). In the present paper the chemical and functional characterization of the fibrils of R. leguminosarum is described. Characterization of purified fibrils by infrared spectroscopy and cellulase treatment followed by thin-layer chromatography showed that the fibrils are composed of cellulose. Purified cellulose fibrils, as well as commercial cellulose, inhibited cap formation when present during the attachment assay. Incubation of the bacteria with purified cellulase just before the attachment assay strongly inhibited cap formation, indicating that the fibrils are directly involved in the attachment process. Tn5-induced fibril-overproducing mutants showed a greatly increased ability to form caps, whereas Tn5-induced fibril-negative mutants lost this ability. None of these Tn5 insertions appeared to be located on the Sym plasmid. Both types of mutants showed normal nodulation properties, indicating that cellulose fibrils are not a prerequisite for successful nodulation under the conditions used. The ability of the fibril-negative mutants to attach to glass was not affected by the mutations, indicating that attachment to pea root hair tips and attachment to glass are (partly) based on different mechanisms. However, growth of the rhizobia under low Ca2+ conditions strongly reduced attachment to glass and also prevented cap formation, although it had no negative effect on fibril synthesis. This phenomenon was found for several Rhizobium spp. It was concluded that both cellulose fibrils and a Ca2+ -dependent adhesin(s) are involved in the attachment of R. leguminosarum to pea root hair tips. A model cap formation as a two-step process is discussed.
Full text
PDF


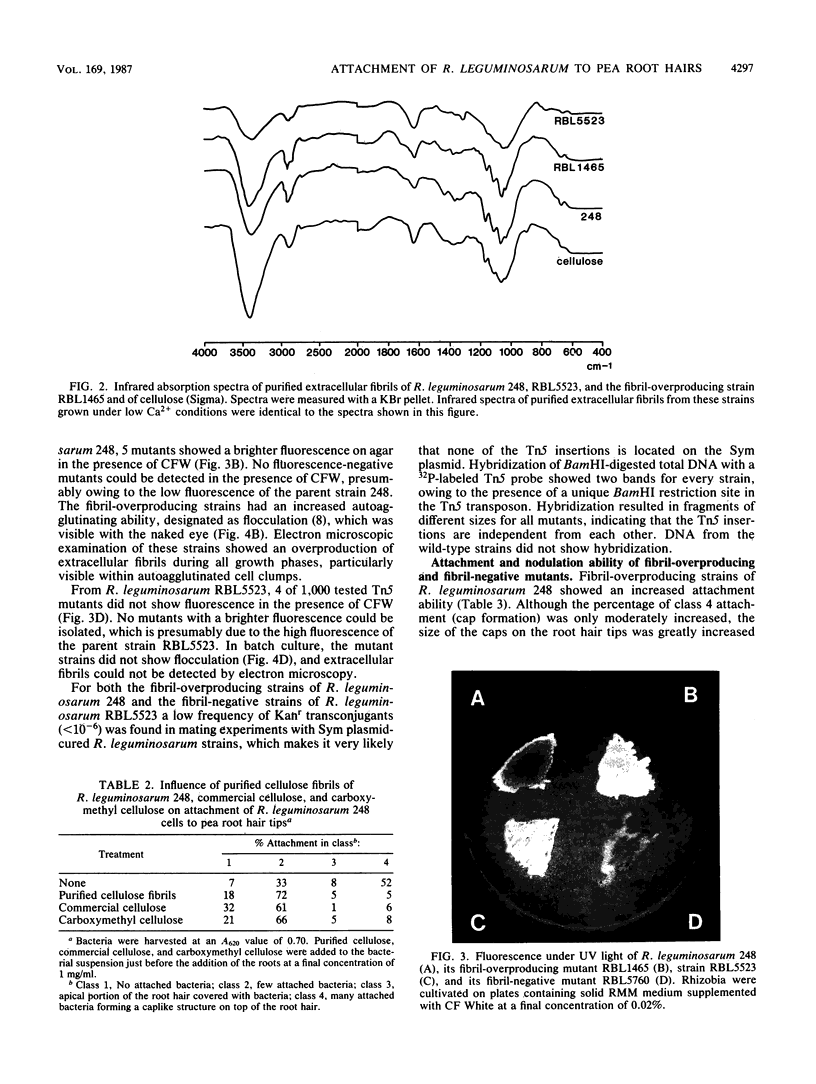
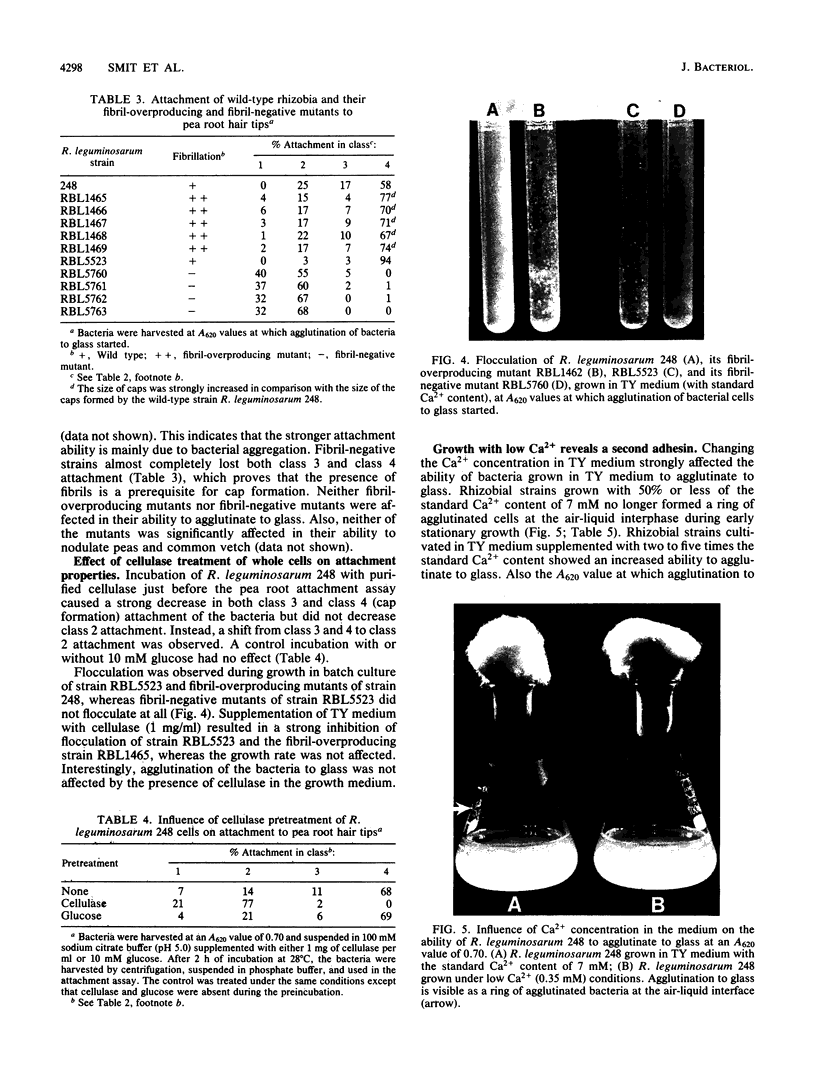

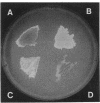
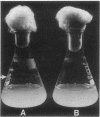
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badenoch-Jones J., Flanders D. J., Rolfe B. G. Association of Rhizobium Strains with Roots of Trifolium repens. Appl Environ Microbiol. 1985 Jun;49(6):1511–1520. doi: 10.1128/aem.49.6.1511-1520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Napoli C. A., Hubbell D. H. Adsorption of bacteria to roots as related to host specificity in the Rhizobium-clover symbiosis. Appl Environ Microbiol. 1976 Jul;32(1):166–171. doi: 10.1128/aem.32.1.166-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Truchet G. L., Sherwood J. E., Hrabak E. M., Abe M., Pankratz S. H. Specific phases of root hair attachment in the Rhizobium trifolii-clover symbiosis. Appl Environ Microbiol. 1984 Dec;48(6):1140–1150. doi: 10.1128/aem.48.6.1140-1150.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinema M. H., Zevenhuizen L. P. Formation of cellulose fibrils by gram-negative bacteria and their role in bacterial flocculation. Arch Mikrobiol. 1971;78(1):42–51. doi: 10.1007/BF00409087. [DOI] [PubMed] [Google Scholar]
- Haahtela K., Tarkka E., Korhonen T. K. Type 1 fimbria-mediated adhesion of enteric bacteria to grass roots. Appl Environ Microbiol. 1985 May;49(5):1182–1185. doi: 10.1128/aem.49.5.1182-1185.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Nurmiaho E. L., Ranta H., Edén C. S. New Method for isolation of immunologically pure pili from Escherichia coli. Infect Immun. 1980 Feb;27(2):569–575. doi: 10.1128/iai.27.2.569-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Matthysse A. G., Holmes K. V., Gurlitz R. H. Elaboration of cellulose fibrils by Agrobacterium tumefaciens during attachment to carrot cells. J Bacteriol. 1981 Jan;145(1):583–595. doi: 10.1128/jb.145.1.583-595.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse A. G. Role of bacterial cellulose fibrils in Agrobacterium tumefaciens infection. J Bacteriol. 1983 May;154(2):906–915. doi: 10.1128/jb.154.2.906-915.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K. K., Bauer W. D. Rhizobium attachment to clover roots. J Cell Sci Suppl. 1985;2:333–345. doi: 10.1242/jcs.1985.supplement_2.18. [DOI] [PubMed] [Google Scholar]
- Napoli C., Dazzo F., Hubbell D. Production of cellulose microfibrils by Rhizobium. Appl Microbiol. 1975 Jul;30(1):123–131. doi: 10.1128/am.30.1.123-131.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek H., Noordermeer I. A., van Sluis C. A., van de Putte P. Expression of the uvrB gene of Escherichia coli: in vitro construction of a pMB9 uvrB plasmid. J Bacteriol. 1978 Feb;133(2):884–896. doi: 10.1128/jb.133.2.884-896.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G., Kijne J. W., Lugtenberg B. J. Correlation between extracellular fibrils and attachment of Rhizobium leguminosarum to pea root hair tips. J Bacteriol. 1986 Nov;168(2):821–827. doi: 10.1128/jb.168.2.821-827.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stacey G., Paau A. S., Brill W. J. Host recognition in the Rhizobium-soybean symbiosis. Plant Physiol. 1980 Oct;66(4):609–614. doi: 10.1104/pp.66.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper S. J., Bauer W. D. Role of Pili (Fimbriae) in Attachment of Bradyrhizobium japonicum to Soybean Roots. Appl Environ Microbiol. 1986 Jul;52(1):134–141. doi: 10.1128/aem.52.1.134-141.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Zurkowski W. Specific adsorption of bacteria to clover root hairs, related to the presence of the plasmid pWZ2 in cells of Rhizobium trifolii. Microbios. 1980;27(107):27–32. [PubMed] [Google Scholar]