Abstract
A mutation within the ompC structural gene of Escherichia coli K-12 which affects expression of outer membrane proteins was characterized. The mutation consisted of a 6-base-pair deletion near the 3' end of the gene which removed the amino acids Val-300 and Gly-301 of the mature coding sequence but otherwise left the reading frame intact. The deletion occurred within a region highly conserved among the porins. No protein product was detected from a single copy of the mutant gene. The mutation caused a trans-dominant decrease in the expression of a wild-type ompC allele. The mutation caused a similar decrease in the amounts of OmpA, OmpF, LamB, and Lc proteins, yet it did not appear to affect the minor outer membrane proteins. It had no significant effect on transcription from either ompF or ompC promoters as measured with lacZ operon fusions. The effects of the mutation on other proteins were completely eliminated when the signal sequence was disrupted so that the mutant protein no longer interacted with the secretion machinery of the cell but instead accumulated as precursor in the cytoplasm. A model is proposed involving the translocation of proteins to the outer membrane and the importance of protein conformation in this process.
Full text
PDF

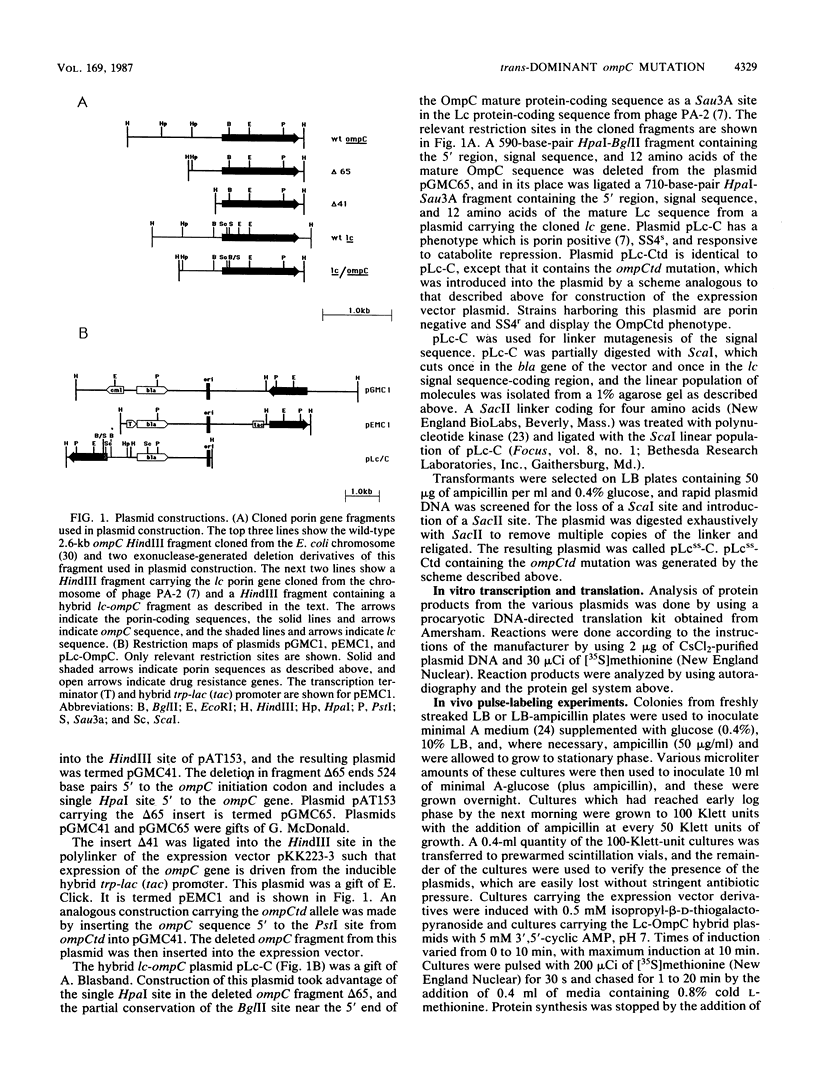


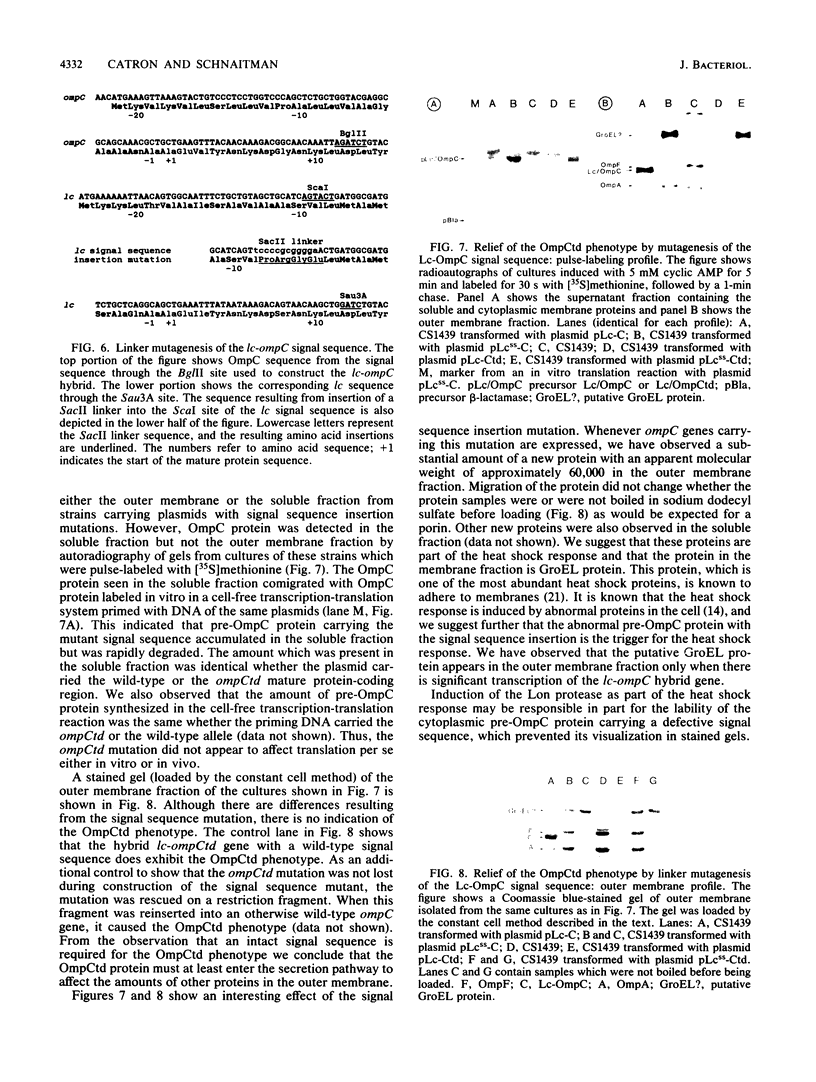


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassford P. J., Jr, Kadner R. J., Schnaitman C. A. Biosynthesis of the outer membrane receptor for vitamin B12, E colicins, and bacteriophage BF23 by Escherichia coli: kinetics of phenotypic expression after the introduction of bfe+ and bfe alleles. J Bacteriol. 1977 Jan;129(1):265–275. doi: 10.1128/jb.129.1.265-275.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S. A., Bremer E., Silhavy T. J. Intragenic regions required for LamB export. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3830–3834. doi: 10.1073/pnas.81.12.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S. A., Hall M. N., Silhavy T. J. Genetic analysis of protein export in Escherichia coli K12. Annu Rev Biochem. 1985;54:101–134. doi: 10.1146/annurev.bi.54.070185.000533. [DOI] [PubMed] [Google Scholar]
- Blasband A. J., Marcotte W. R., Jr, Schnaitman C. A. Structure of the lc and nmpC outer membrane porin protein genes of lambdoid bacteriophage. J Biol Chem. 1986 Sep 25;261(27):12723–12732. [PubMed] [Google Scholar]
- Bosch D., Leunissen J., Verbakel J., de Jong M., van Erp H., Tommassen J. Periplasmic accumulation of truncated forms of outer-membrane PhoE protein of Escherichia coli K-12. J Mol Biol. 1986 Jun 5;189(3):449–455. doi: 10.1016/0022-2836(86)90316-5. [DOI] [PubMed] [Google Scholar]
- Chen L., Tai P. C., Briggs M. S., Gierasch L. M. Protein translocation into Escherichia coli membrane vesicles is inhibited by functional synthetic signal peptides. J Biol Chem. 1987 Feb 5;262(4):1427–1429. [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Freudl R., Schwarz H., Degen M., Henning U. The signal sequence suffices to direct export of outer membrane protein OmpA of Escherichia coli K-12. J Bacteriol. 1987 Jan;169(1):66–71. doi: 10.1128/jb.169.1.66-71.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudl R., Schwarz H., Klose M., Movva N. R., Henning U. The nature of information, required for export and sorting, present within the outer membrane protein OmpA of Escherichia coli K-12. EMBO J. 1985 Dec 16;4(13A):3593–3598. doi: 10.1002/j.1460-2075.1985.tb04122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. A., Goldberg A. L. Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell. 1985 Jun;41(2):587–595. doi: 10.1016/s0092-8674(85)80031-3. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the major outer membrane proteins of Escherichia coli. Annu Rev Genet. 1981;15:91–142. doi: 10.1146/annurev.ge.15.120181.000515. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R., Boos W. Translational control of exported proteins in Escherichia coli. J Bacteriol. 1986 Aug;167(2):462–466. doi: 10.1128/jb.167.2.462-466.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge R., Boos W. Defective secretion of maltose- and ribose-binding proteins caused by a truncated periplasmic protein in Escherichia coli. J Bacteriol. 1985 Jun;162(3):972–978. doi: 10.1128/jb.162.3.972-978.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. E., Pratt J. M., Stoker N. G., Holland I. B. An inner membrane protein N-terminal signal sequence is able to promote efficient localisation of an outer membrane protein in Escherichia coli. EMBO J. 1985 Sep;4(9):2377–2383. doi: 10.1002/j.1460-2075.1985.tb03942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Translocation of phospholipids between the outer and inner membranes of Salmonella typhimurium. J Biol Chem. 1977 Oct 25;252(20):7405–7412. [PubMed] [Google Scholar]
- Krueger J. H., Walker G. C. groEL and dnaK genes of Escherichia coli are induced by UV irradiation and nalidixic acid in an htpR+-dependent fashion. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1499–1503. doi: 10.1073/pnas.81.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus M. R., van Renswoude J., Harford J. B., Chang E. H., Carty R. P., Klausner R. D. Prediction of the three-dimensional structure of the transforming region of the EJ/T24 human bladder oncogene product and its normal cellular homologue. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5253–5257. doi: 10.1073/pnas.80.17.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Identification of three genes controlling production of new outer membrane pore proteins in Escherichia coli K-12. J Bacteriol. 1978 Sep;135(3):1118–1129. doi: 10.1128/jb.135.3.1118-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Outer membrane proteins of Escherichia coli. VII. Evidence that bacteriophage-directed protein 2 functions as a pore. J Bacteriol. 1978 Mar;133(3):1181–1189. doi: 10.1128/jb.133.3.1181-1189.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A., McDonald G. A. Regulation of outer membrane protein synthesis in Escherichia coli K-12: deletion of ompC affects expression of the OmpF protein. J Bacteriol. 1984 Aug;159(2):555–563. doi: 10.1128/jb.159.2.555-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., van Tol H., Lugtenberg B. The ultimate localization of an outer membrane protein of Escherichia coli K-12 is not determined by the signal sequence. EMBO J. 1983;2(8):1275–1279. doi: 10.1002/j.1460-2075.1983.tb01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Gilmore R., Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984 Aug;38(1):5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]







