Abstract
Group I metabotropic glutamate receptors (mGluRs) are implicated in diverse processes such as learning, memory, epilepsy, pain and neuronal death. By inhibiting background K+ channels, group I mGluRs mediate slow and long-lasting excitation. The main neuronal representatives of this K+ channel family (K2P or KCNK) are TASK and TREK. Here, we show that in cerebellar granule cells and in heterologous expression systems, activation of group I mGluRs inhibits TASK and TREK channels. d-myo-inositol-1,4,5-triphosphate and phosphatidyl-4,5-inositol-biphosphate depletion are involved in TASK channel inhibition, whereas diacylglycerols and phosphatidic acids directly inhibit TREK channels. Mechanisms described here with group I mGluRs will also probably stand for many other receptors of hormones and neurotransmitters.
Keywords: diacylglycerol/glutamate/IP3/phosphatidic acid/PIP2
Introduction
Glutamate is a major excitatory neurotransmitter in the central nervous system. To mediate fast excitatory transmission, glutamate activates postsynaptic ionotropic receptors. Glutamate also activates metabotropic receptors (mGluRs) (Sladeczek et al., 1985; Sugiyama et al., 1987), which modulate neuronal excitability and synaptic transmission, resulting in a slow depolarization and increase in cell firing (Pin and Duvoisin, 1995). There are currently eight subtypes of mGluRs (mGluR1–8) and multiple splice variants (Conn and Pin, 1997). Group II and III receptors are coupled to Gi, leading to a decrease of cAMP. Group II and III mGluRs (except mGluR6) predominate in presynaptic elements, where they regulate the release of glutamate and other neurotransmitters. Conversely, group I mGluRs (mGluR1 and mGluR5) are coupled to Gq and stimulate the phospholipase C (PLC) pathway, leading to increased phosphoinositide hydrolysis (Conn and Pin, 1997). They are located essentially in postsynaptic areas, where they contribute to the enhancement of cellular excitability via interactions with other postsynaptic processes including background potassium (K+) current inhibition (Charpak et al., 1990; Glaum and Miller, 1992; McCormick and von Krosigk, 1992; Guerineau et al., 1994; Pin and Duvoisin, 1995).
Background or ‘leak’ K+ currents are central to electrical excitability by controlling the resting membrane potential and cell membrane resistance. Inhibition of these background K+ currents by several neurotransmitters induces membrane depolarization and subsequent action potential firing, as well as an increase in cell membrane resistance amplifying responses of synaptic inputs (Charpak et al., 1990; Nicoll et al., 1990; Glaum and Miller, 1992; McCormick and von Krosigk, 1992; Guerineau et al., 1994; Pin and Duvoisin, 1995; Millar et al., 2000; Talley et al., 2000). In contrast, their opening by volatile anesthetics contributes to neuronal hyperpolarization leading to analgesia and immobilization (Patel et al., 1999). Background K+ channels have two pore domains (K2P) and four transmembrane segments (Lesage and Lazdunski, 2000; Patel and Honore, 2001a). In the nervous system, important representatives of this family are the TASK and TREK channels (Fink et al., 1996; Duprat et al., 1997; Lauritzen et al., 2000; Lesage and Lazdunski, 2000; Talley et al., 2001). They are relatively insensitive to the broad-spectrum K+ channel blockers (tetraethylammonium, 4-aminopyridine, Cs+ and Ba2+) but their activities are increased by volatile anesthetics (Patel et al., 1999; Patel and Honore, 2001b). TREK channels (TREK1 and TREK2) are the targets of many physico-chemical stimuli, including internal pH, temperature, osmolarity and membrane stretch (Patel et al., 1998; Lesage et al., 2000; Patel and Honore, 2001a). TREK1 and TREK2 channels are potently activated by polyunsaturated fatty acids and lysophospholipids, and inhibited by neurotransmitters that increase cAMP, via a protein kinase A-dependent phosphorylation process (Patel et al., 1998; Lesage et al., 2000) and by Gq-coupled receptors via an unknown mechanism (Lesage et al., 2000). TASK channels (TASK1 and TASK3) are highly sensitive to small pH variations near physiological pH and to hypoxia (Duprat et al., 1997; Buckler et al., 2000; Kim et al., 2000; Patel and Honore, 2001a). These channels are also modulated by various neurotransmitters and hormones but the precise transduction mechanism through which these molecules exert their control is still unknown (Millar et al., 2000; Talley et al., 2000).
In this study, we show that by acting on group I Gq-coupled metabotropic receptors, glutamate strongly inhibits both TASK and TREK channels using two distinct pathways. TASK1 and TASK3 currents are inhibited by phosphatidyl-4,5-inositol-biphosphate (PIP2) depletion and d-myo-inositol-1,4,5-triphosphate (IP3) liberation following PLC activation, while TREK1 and TREK2 currents are directly blocked by diacylglycerols (DAG) and phosphatidic acids (PA).
Results
Group I metabotropic glutamate receptors inhibit TASK and TREK background potassium channels
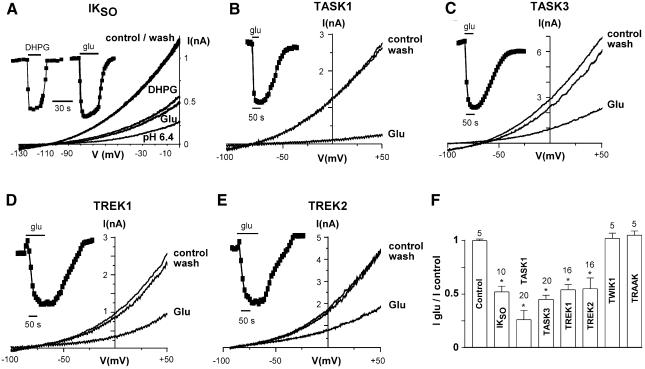
Cerebellar granule neurons express both background K+ currents (named IKSO, for standing outward; Millar et al., 2000; Brickley et al., 2001; Han et al., 2002) and group I mGluRs (Ango et al., 2001). Using the perforated patch technique, we recorded robust K+ currents with a mean amplitude of 1.16 ± 0.16 nA (n = 14) at 0 mV in control condition (Figure 1A). Application of glutamate reversibly inhibited IKSO at all tested potentials, showing no voltage dependence (Figure 1A and F). The inhibition was reproduced by 100 µM (RS)-3,5-dihydroxyphenylglycine (DHPG; 49.7 ± 5% inhibition, n = 14; Figure 1A) indicating the involvement of group I mGluRs (mainly mGluR1a; Ango et al., 2001). IKSO is very sensitive to extracellular acidification (72 ± 3% inhibition, n = 14, pH 6.4; Figure 1A) indicating the involvement of TASK channels (Duprat et al., 1997; Kim et al., 2000). Furthermore, IKSO is blocked by 5 µM ruthenium red (76 ± 2% inhibition, n = 4), but is insensitive to 3 µM methanandamide (14 ± 16% inhibition, n = 4), demonstrating a major contribution of TASK3 currents (Maingret et al., 2001; Czirjak and Enyedi, 2002; Lauritzen et al., 2003). However, IKSO is also generated by other K2P channels, mainly TASK1 and TREK2 (Millar et al., 2000; Han et al., 2002). Thus, these channels were co-expressed in COS-7 cells with mGluR1a and tested for their glutamate sensitivity. As observed with IKSO, glutamate reversibly inhibited TASK1, TASK3, TREK1 and TREK2 currents (Figure 1B–F), whereas no effect was observed on TASK2 (n = 5; data not shown), TWIK1 and TRAAK currents (Figure 1F), or in COS-7 cells lacking mGluR1a (n = 5; data not shown) and COS-7 cells expressing only mGluR1a (control, Figure 1F). This lack of effect on TWIK-1 and TRAAK channels is interesting since they are both highly expressed in the brain (Lesage et al., 1996; Fink et al., 1998; Lauritzen et al., 2000; Talley et al., 2001). Similar glutamate effects on TREK and TASK channels were obtained using COS-7 cells containing mGluR5 (n = 12; data not shown).
Fig. 1. Glutamate inhibits background potassium currents in native neurons and in heterologous expression systems. (A) Sustained outward potassium currents (IKSO) recorded in cerebellar granule neurons before and after 100 µM glutamate, 100 µM DHPG, pH 6.4, application. The time-course of glutamate and DHPG inhibition (measured at 0 mV) is indicated as an inset. (B–E) Glutamate (50 µM) inhibits recombinant TASK1 (B), TASK3 (C), TREK1 (D) and TREK2 (E) channels as expressed in COS-7 cells containing mGLUR1a receptors. Currents were elicited by voltage ramp (1 mV/7 ms) ranging between indicated potentials and applied every 10 s. (F) Percent of current inhibition induced by glutamate (measured at 0 mV) on various K2P channels expressed in brain. *P < 0.01.
Glutamate inhibition displayed faster kinetics for TASK compared with TREK currents (P < 0.01; Figure 1B–E), suggesting distinct mechanisms of inhibition. Moreover, TREK inhibition was often preceded by small increases of the currents after glutamate application (+7 ± 2%, n = 16 for TREK1 currents and +8 ± 2%, n = 16 for TREK2 currents; Figure 1D and E).
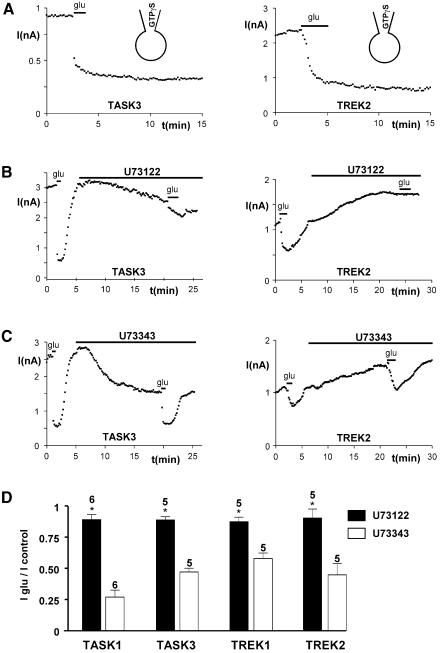
Gq/11 and PLC are involved in TASK and TREK inhibition independently of calcium release and protein kinase C activation
Glutamatergic mGluR1 receptors usually couple via the G proteins Gq/11 and PLC (Sladeczek et al., 1985; Sugiyama et al., 1987). Therefore, we tested whether TASK and TREK currents are modulated via this pathway. In the presence of 1 mM GTPγS in the patch-pipette, glutamate induced an irreversible inhibition of TASK3 and TREK2 currents (<5% of wash, n = 5; P < 0.01; Figure 2A). To simplify, and because IKSO comprises mainly TASK3 and TREK2 (Han et al., 2002), all further presented data relate to these channels. The same results were obtained with TASK1 and TREK1 (n = 5, P < 0.01; data not shown). Neither TASK nor TREK inhibition was affected after 16–24 h of treatment with 200 ng/ml pertussis toxin (n = 20; data not shown) or 500 ng/ml cholera toxin (n = 10; data not shown). Furthermore, the PLC inhibitor U73122 (5 µM) perfused for 15 min, i.e. in conditions similar to those which have been used to establish the PLC-mediated inhibition of IM (KCNQ) currents (Suh and Hille, 2002), suppressed TASK and TREK inhibitions by glutamate. U73122 also induced a down-regulation of TASK currents but an up-regulation of TREK currents (Figure 2B). These effects are probably unrelated to the action of U73122 on PLC, since they are reproduced by a U73122 structural analog, U73343, which is inactive on PLC. Importantly, unlike U73122, U73343 had no effect on TASK and TREK current inhibitions by glutamate (Figure 2B–D). Activation of PLC leads to the cleavage of PIP2 into two second messengers, the membrane-bound DAG that activates protein kinase C (PKC) and the soluble IP3 that mobilizes Ca2+ from intracellular stores via fixation to IP3 receptors. Buffering intracellular Ca2+ concentration to 10 nM or 5 µM with EGTA did not significantly affect TASK and TREK currents or their inhibition by glutamate (variation/control = 10 ± 12%, n = 10 for TASK currents; 15 ± 14%, n = 10 for TREK currents). The same results were obtained with the more potent calcium chelator BAPTA (used at 5 mM) on TASK and TREK currents (n = 8; not shown, but see Figure 4C). To explore the potential role of PKC under our conditions, we pre-treated cells expressing TASK or TREK currents with 100 nM staurosporine, a broad-spectrum kinase inhibitor. Stauro sporine did not prevent TASK and TREK current inhibitions by glutamate (variation/control = 14 ± 16%, n = 6 for TASK currents; 16 ± 13%, n = 6 for TREK currents). The same results were obtained in the presence of 50 µM PKC peptide inhibitor (pseudosubstrate fragment 19–36) in the patch pipette (n = 12; data not shown). Similarly, the PKC activator phorbol 12,13-dibutyrate (PdBU; 5 µM perfused 15 min) did not significantly affect TASK and TREK currents or their inhibition by glutamate (variation/control = 8 ± 17%, n = 11 for TASK currents; 14 ± 16%, n = 13 for TREK currents). The same results were obtained using 100 nM phorbol 12-myristate 13-acetate (PMA; n = 9; data not shown). The absence of effect of the generic mediators of Gq/11-coupled receptors lead us to turn our investigation to the possibility of direct roles of PIP2 and its downstream products IP3 and DAG.
Fig. 2. Glutamate inhibition of background potassium currents involved G proteins and PLC. (A) Recovery of TASK3 and TREK2 currents in the presence of 1 mM GTPγS in the patch–clamp pipette. (B and C) Glutamate inhibition of TASK3 and TREK2 currents before and after 15 min treatment with 5 µM PLC inhibitor U73122 (B) or its inactive analog U73343 (C). (D) Summary of the effects produced by U73122 (black bars) and U73343 (white bars). *P < 0.01.
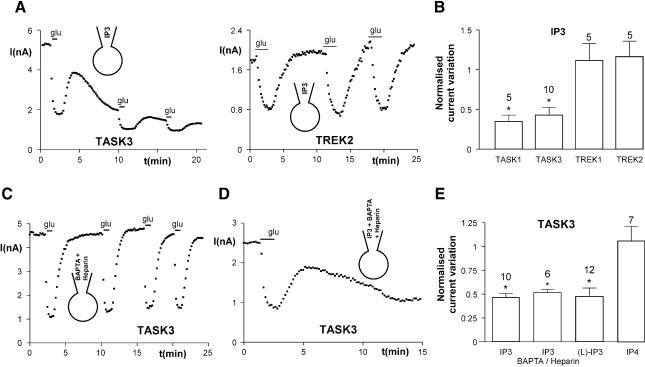
Fig. 4. IP3 inhibits TASK currents independently of IP3 receptors. (A) Inhibition of TASK3 currents by 50 µM IP3 added in the patch–clamp pipette. (B) Summary of the effects of IP3 application (10 min). (C) Inhibition of TASK3 currents by glutamate in the presence of 5 mM BAPTA and 5 mg/ml heparin added in the patch–clamp pipette. (D) Inhibition of TASK3 currents by 50 µM IP3 in the presence of 5 mM BAPTA and 5 mg/ml heparin. (E) The pharmacologically inactive and non-metabolizable analog l-myo-Ins(1,4,5)P3 (50 µM) inhibits TASK3 currents, while Ins(1,3,4,5)P4 (50 µM) has no effect. *P < 0.01.
Depletion of PIP2 is involved in glutamate-mediated TASK inhibition
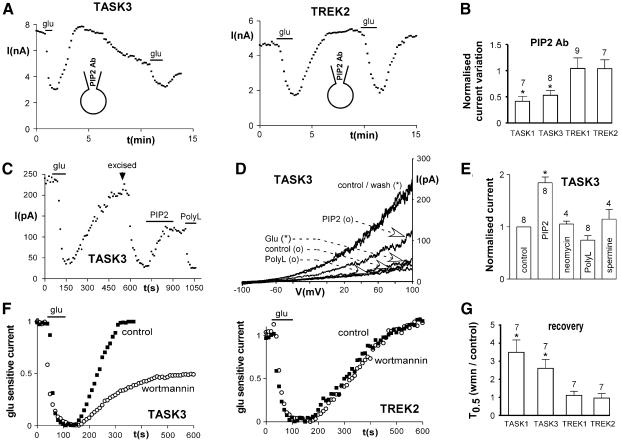
Monoclonal antibodies against PIP2 have been used recently by other authors to analyze the regulatory function of PIP2 on ion channels or transporters (Chuang et al., 2001; Runnels et al., 2002; Wu et al., 2002). The addition of anti-PIP2 (1:50) in the patch–clamp pipette induced a strong run-down of both TASK1 and TASK3 currents (Figure 3A and B) without effect on TREK currents. In contrast, an unrelated IgM antibody (1:50) was ineffective (n = 6; data not shown). We then tested whether PIP2 could directly modulate TASK currents in inside-out patches. In the cell-attached mode, glutamate potently inhibited TASK3 currents (56 ± 9.7% inhibition, n = 4; Figure 3C and D). After excision to the inside-out mode, TASK3 currents strongly decreased (run-down of current: 57 ± 6.6%, n = 8; Figure 3C and D). Bath application of the naturally occurring PIP2 (1-stearoyl-2-arachidonoyl-sn-glycero-3-phospho-1-d-myo-inositol-4,5-bisphosphate, 25 µM) in inside-out patches increased TASK3 currents by 84% (Figure 3C–E). Application of poly-cationic molecules, such as poly-lysine, neomycin and spermine, which act as PIP2 scavengers (Huang et al., 1998), resulted in a fast and complete block of the current, following reactivation by PIP2 (Figure 3C–E). Despite the persistence of the current block upon poly-lysine wash-out, a second application of PIP2 fully reactivated TASK3 currents (n = 4; data not shown). Therefore, PIP2 appears to be necessary to maintain TASK channels active, which suggests strongly that depletion of PIP2 could be involved in glutamate-mediated inhibition of TASK currents. The replenishment of PIP2 requires lipid kinases, especially the phosphatidylinositol-4-OH kinase [PI(4)K]. Application of wortmannin at concentrations 100-fold higher than those used to inhibit PI(3)K (10–100 nM) decreases replenishment of PIP2 after receptor-mediated depletion through inhibition of PI(4)K (Nakanishi et al., 1995). In the whole-cell configuration, perfusion of 10 µM wortmannin for 5 min decreased TASK currents (29 ± 6% inhibition, n = 11, and 27 ± 5% inhibition, n = 11, for TASK1 and TASK3 currents, respectively; P < 0.01) and slowed their recovery following glutamate inhibition (Figure 3C and D) without effect on TREK currents. These results were not due to inhibition of PI(3)K, since 100 nM wortmannin was ineffective (n = 5; data not shown). Furthermore, we excluded a direct block of TASK currents by wortmannin, since 10 µM wortmannin had no effect in inside-out patches expressing TASK3 currents, even after PIP2 application (n = 2; data not shown). Also, wortmannin did not produce run-down of TASK3 currents in the whole-cell mode after their inhibition by PIP2 antibodies (n = 3; data not shown). Interestingly, recovery of TASK currents after glutamate application was not completely blocked by 50 µM wortmannin (percent recovery: 31 ± 13%, n = 7, and 38 ± 18%, n = 6, for TASK1 and TASK3 currents, respectively), suggesting that although PIP2 depletion is involved in inhibition of TASK currents, another mechanism also contributes to glutamate mediated inhibition.
Fig. 3. PIP2 depletion is involved in TASK current inhibition. (A) Addition of PIP2 antibody (1:50) in the patch–clamp pipette inhibits TASK3 currents without effect on TREK2 currents. (B) Summary of the effects of PIP2 antibody application (10 min). (C) Time-course of inhibition of TASK3 currents by glutamate (measured at +100 mV) in the cell-attached configuration. After excision to the inside-out configuration (arrow), PIP2 (25 µM) was applied after current run-down. Poly-lysine (30 µg/ml) inhibits PIP2-stimulated currents. (D) TASK3 currents obtained with voltage-ramps from the same cell as in (C). (*), Cell-attached mode; (o), inside-out mode. (E) Summary of the effects of 25 µM PIP2 and of 100 µM neomycin, 30 µg/ml poly-lysine or 100 µM spermine applied after 25 µM PIP2 as in (C). (F) Inhibition of PIP2 synthesis by 10 µM wortmannin (perfused during 5 min) slows down the recovery of TASK currents without effect on TREK currents (black squares, first glutamate application in control conditions; white circles, second glutamate application during wortmannin perfusion). (G) Effects of wortmannin on current recovery, quantified as the time for 50% recovery after glutamate application (T0.5). *P < 0.01.
IP3 inhibits TASK currents independently of IP3 receptors
We then investigated the role of IP3 in glutamate-mediated current inhibition. The addition of IP3 in the patch–clamp pipette (50 µM) strongly decreased TASK currents (Figure 4A and B) with no effect on TREK. However, glutamate could still inhibit residual TASK currents despite the presence of IP3, which is consistent with a PIP2 depletion-mediated inhibition. Glutamate effects (54 ± 4% inhibition, n = 5; Figure 4C) persisted in the presence of heparin (5 mg/ml), which inhibit IP3 receptors, and BAPTA (5 mM), which prevents increase in intracellular calcium. IP3 receptors are not involved in the IP3-mediated inhibition of the TASK currents since IP3 could still inhibit TASK3 in the presence of BAPTA and heparin (Figure 4D and E). Application of 50 µM IP3 to inside-out patches expressing TASK3 currents had no effect, even after PIP2 application and in the presence or absence of 5 µM Ca2+ (n = 8; data not shown). The effects of IP3 are independent of its metabolism, since its non-metabolizable and IP3 receptor inactive analog l-myo-Ins(1,4,5)P3 (Willcocks et al., 1987; Hirata et al., 1990) also potently inhibits TASK3 currents in the whole-cell configuration (Figure 4E). IP3-mediated TASK current inhibition is not due to unspecific interaction with charged phosphate groups, since Ins(1,3,4,5)P4 had no effect (Figure 4E). Taken together, these results indicate that TASK currents are inhibited directly by depletion of PIP2 and indirectly by IP3, whereas PLC-induced inhibition of TREK currents, under the conditions used in this work, does not involve intracellular Ca2+, PKC activation, PIP2 or IP3. Therefore, we then explored whether DAG or its metabolites could be involved in the inhibition of TREK currents, as previously observed in the activation of TRPC3 and TRPC6 by histamine (Hofmann et al., 1999).
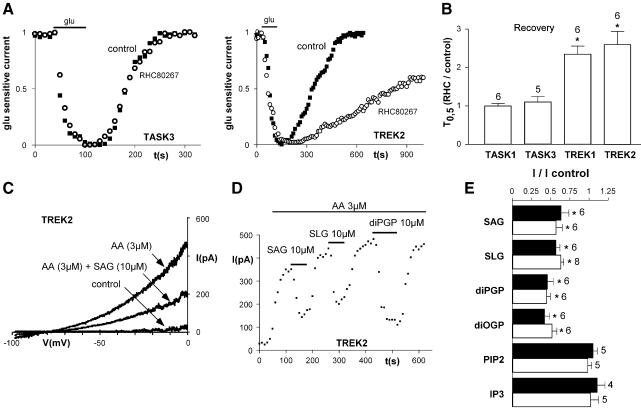
DAG and PA inhibit TREK currents
To assess the role of DAG, we first measured TREK currents during application of the membrane-permeable DAG analog 1,2-dioctanoyl-sn-glycerol (DOG). DOG (50 µM) rapidly decreased TREK currents (percent inhibition of TREK2 currents after 3 min of perfusion: 54 ± 17%, n = 10, P < 0.01). In mammalian cells DAG is mainly metabolized by DAG lipase. If DAG is implicated in glutamate-mediated TREK inhibition, then the block of DAG lipase should slow the recovery of TREK currents after glutamate application. Indeed, the DAG lipase inhibitor 1,6-bis (cyclohexyloximinocarbonyl-amino) hexane (RHC80267) slowed the recovery of TREK currents (Figure 5A and B) without effect on TASK currents. This result prompted us to investigate whether DAG or its phosphorylated metabolite PAs, which are mainly generated from DAG via DAG kinase or from phospholipids via phospholipase D, could directly inhibit TREK currents. Various physiological DAGs and PAs were applied to inside-out patches expressing TREK currents. In inside-out patches, TREK currents only display a small activity, but this activity can be potently stimulated by acidic pH, negative pressures or in the presence of polyunsaturated fatty acids such as arachidonic acid (AA) (Patel et al., 1998; Lesage et al., 2000; Patel and Honore, 2001a). Strong TREK currents were obtained in the presence of AA (Figure 5C and D). Under these conditions, application of the endogenous impermeable DAG analogs 1-stearoyl-2-arachidonoyl-sn-glycerol (SAG, 10 µM) or 1-stearoyl-2-linoleoyl-sn-glycerol (SLG, 10 µM) to the internal side of the membrane patch reversibly inhibited TREK1 and TREK2 currents (Figure 5D–F). Interestingly, as observed with DAG, PA analogs, 1,2-dipalmitoyl-sn-glycerol-3-phosphate (diPGP 10 µM) and 1,2-dioleoyl-sn-glycerol-3-phosphate (diOGP 10 µM), strongly decreased TREK currents (Figure 5D and E). The inhibitory effects of DAG and PA analogs were not observed in inside-out patches expressing TASK currents, even after reactivation with PIP2 (n = 5; data not shown). In agreement with the results of the whole-cell experiments, we observed that neither PIP2 nor IP3 (in the presence or absence of 5 µM Ca2+) altered AA-activated TREK2 currents in inside-out patch configurations (Figure 5E).
Fig. 5. DAGs and PAs inhibit TREK currents. (A and B) Fifteen minute perfusion of 50 µM RHC80267 (rhc), a DAG lipase inhibitor, slows down the recovery of TREK currents without effect on TASK currents (black squares, first glutamate application in control conditions; white circles, second glutamate application during RHC80267 application). (C) Direct inhibition of TREK2 currents by 10 µM SAG in inside-out patch configuration. TREK2 currents are activated by 3 µM AA. (D) Time-course of TREK2 current inhibition by 10 µM SAG, SLG and diPGP. (E) Percent of inhibition of various DAGs and PAs and phosphoinositides in the presence of 3 mM internal Mg2+, on TREK1 (black bars) and TREK2 (white bars). *P < 0.01.
Discussion
Various neurotransmitters induce slow excitation in diverse regions of the CNS by inhibiting background K+ currents (Charpak et al., 1990; Nicoll et al., 1990; Glaum and Miller, 1992; McCormick and von Krosigk, 1992; Guerineau et al., 1994; Pin and Duvoisin, 1995; Millar et al., 2000; Talley et al., 2000). K2P channels are now known to be major contributors of background K+ current (Lesage and Lazdunski, 2000). Among these K2P channels, TASK and TREK channels have been recently shown to mediate neurotransmitter-sensitive background K+ currents in cerebellar granule cells, hypoglossal motoneurons or in adrenal cortex (Millar et al., 2000; Talley et al., 2000; Czirjak et al., 2001; Enyeart et al., 2002). This study shows that mGluR1 glutamate receptors inhibit both K+ background currents in cerebellar granule cells and heterologously expressed TASK and TREK channels. Activation of mGluR1 receptors has no effect on other K2P channels that are abundantly expressed in the brain such as TWIK-1 and TRAAK (Lesage et al., 1996; Fink et al., 1998; Lauritzen et al., 2000; Talley et al., 2001).
It was known that Gq-coupled receptors inhibit background K+ currents (Lesage et al., 2000; Millar et al., 2000; Talley et al., 2000; Czirjak et al., 2001); however, the signaling elements involved in the transduction cascades were unknown. We have now shown that inhibition of TASK1, TASK3, TREK1 and TREK2 by glutamate via mGluR1 receptors requires PTX-insensitive G proteins coupled to PLC. If inhibition of both TASK and TREK channels requires PLC activation, the downstream molecular events leading to TASK and TREK inhibition are clearly different (Figure 6). TASK1 and TASK3 currents are inhibited by PIP2 depletion following PLC activation. This effect is mimicked by PIP2 antibodies, while PIP2 application to inside-out patches reactivates TASK currents after run-down. Inhibition of PIP2 replenishment induced run-down of TASK currents and slowed their recovery after glutamate inhibition. Thus, TASK channels belong to the growing family of proteins, including several transporters (Hilgemann et al., 2001) and ion channels such as the inward rectifier potassium channels (KATP, IRK, GIRK and ROMK), M-type channels (KCNQ2/KCNQ3), epithelial sodium channels (ENaC), transient receptor potential (TRPL, TRPM7, VR1) and calcium channels (α1A, α1B), that are regulated by variations of the PIP2 level (Huang et al., 1998; Chuang et al., 2001; Hilgemann et al., 2001; Runnels et al., 2002; Suh and Hille, 2002; Wu et al., 2002). However, we have also shown that IP3 drastically decreases TASK currents. The effect is independent of IP3 metabolism but is indirect, since IP3 had no effect on inside-out patches expressing TASK channels. We have no definite mechanism for the IP3 inhibition of TASK currents but we suggest that IP3 might modulate PIP2 metabolism by acting as a feed-back molecule on phospholipases or kinases. Therefore, the IP3 regulation of TASK currents would be via PIP2 levels. Regulation of TASK channels involves first the depletion of membrane-bound PIP2 and second the liberation of its soluble metabolite IP3.
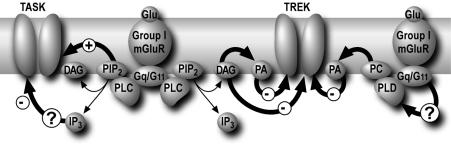
Fig. 6. A model of TASK and TREK modulation by group I mGluRs. Activation of PLC by group I Gq-coupled metabotropic receptors inhibit both TASK (TASK1 and TASK3) and TREK (TREK1 and TREK2) channels using two distinct pathways. TASK channels are inhibited directly by PIP2 depletion and by IP3 via an as yet unknown (?) mechanism. In contrast, TREK channels are directly blocked by DAGs independently of PKC activation. In addition, PA, which is mainly generated from DAG via DAG kinase or from phospholipids [mainly phosphatidylcholine (PC)] via phospholipase D (PLD), is a direct blocker of TREK channels.
In contrast, PLC activation inhibits TREK1 and TREK2 currents via DAGs independently of PKC activation. The DAG inhibition is most likely direct since it has been observed in excised patches expressing both TREK1 and TREK2 channels. Therefore, the recovery of glutamate inhibition via the mGluR1 receptor is delayed when DAG metabolism is blocked in the presence of a DAG lipase inhibitor. Interestingly, PAs are also direct inhibitors of both TREK1 and TREK2 channels, suggesting that PLD activation also modulates TREK channels activities. Many molecular stimuli activate PLD, including activation of Gq-coupled receptors, which comprise mGluR1 (Klein et al., 1997). Therefore, inhibition of TREK currents depends on both hydrolysis of phosphatidylinositol by PLC and other phospholipids (mainly phosphatidylcholine) by PLD (Figure 6). To our knowledge, TREK1 and TREK2 represent the first class of K+ channels directly modulated by both DAGs and PAs. Our work also shows for the first time a role of PAs in the regulation of ionic channels.
Group I mGluRs are postsynaptic and enhance cellular excitability through different mechanisms, including background K+ current inhibition (Charpak et al., 1990; Glaum and Miller, 1992; McCormick and von Krosigk, 1992; Guerineau et al., 1994; Pin and Duvoisin, 1995). Group I mGluR1 and mGluR5 are both present in several CNS structures such as hippocampus, cortex, thalamus and cerebellum, where they are involved in many brain functions (Bordi and Ugolini, 1999). In the cerebellum, mGluR1 plays a key role in motor learning and motor coordination (Aiba et al., 1994; Conquet et al., 1994), whereas mGluR5 in the hippocampus contributes to the induction of long-term potentiation and associative learning (Anwyl, 1999). Group I mGluRs are also implicated in a variety of disorders including epilepsy, ischemia, nociception and neurodegenerative diseases (Bordi and Ugolini, 1999). In pathological states, such as ischemia, overstimulation of glutamate receptors is excitotoxic and results in neuronal death (Choi, 1988). Group I mGluRs agonists amplify the excitotoxic neuronal degeneration induced by NMDA in cultured murine cortical cells, whereas antagonists are neuroprotectors (Nicoletti et al., 1996). On the other hand, TREK channels are activated by a neuroprotective agent such as riluzole, and also by polyunsaturated fatty acids and lysophospholipids (Patel et al., 1998; Duprat et al., 2000; Lauritzen et al., 2000; Lesage et al., 2000; Patel and Honore, 2001a), which are potent protectors against global ischemia and epilepsy (Hibbeln, 1998; Lauritzen et al., 2000). They are also neuroprotective in in vitro models of excitotoxicity using primary cultures of cerebellar granule cells (Blondeau et al., 2002). The inhibition of TASK and TREK currents by glutamate via group I metabotropic receptors provides a molecular mechanism by which glutamate induces cellular excitability, but also probably overexcitability, leading to neuronal death.
Both TASK and TREK channels are the targets of many regulatory processes that are commanded via intracellular (TREK) or extracellular (TASK) pH variations, hypoxia (TASK), osmolarity and membrane stretch (TREK). On the other hand, these K2P channels now appear as central targets of transduction pathways including PIP2, IP3, DAG and PA. This sophistication of regulation is uncommon in the K+ channel family and is reminiscent of that observed in the TRP family (Kiselyov and Muallem, 1999).
Materials and methods
Whole-cell recordings in rat cerebellar granule neurons
Primary cultures of cerebellar granule neurons were prepared from 6- to 8-day-old Wistar rats (Iffacredo, France). Cells were plated at a density of 2 × 106 cells per 35 mm culture dish. Eight to 12 days after plating, perforated patch measurements were obtained using amphotericin B added to the pipette solution to a final concentration of 240 µg/ml. The pipette solution contained (in mM): 125 KCl, 5 MgCl2, 0.1 BAPTA and 5 HEPES at pH 7.4 with KOH; and the bath solution contained (in mM): 135 NaCl, 2.5 KCl, 2 MgCl2, 0.5 CaCl2, and 10 HEPES at pH 7.4 with NaOH. Whole-cell recordings were performed at room temperature using a RK400 patch–clamp amplifier (Bio-Logic, Grenoble, France). Data were analysed using pClamp software. All results are expressed as mean ± SEM, with n indicating the number of cells tested.
Electrophysiology in transfected COS cells
COS cells were seeded at a density of 20 000 cells per 35 mm culture dish, 24 h before transfection. Cells were transfected by the classical DEAE-dextran method with 1 µg of pIRES-CD8 containing hTASK1, hTASK3, hTREK1 or hTREK2, with or without 1 µg of pRK/mGluR1a or pRK/mGluR5a expression vectors (a generous gift of Drs J.P.Pin and A.Dumuis). Transfected cells were visualized 48 h after transfection using anti-CD8 antibody-coated beads. For whole-cell experiments, the pipette solution (INT) contained (in mM):155 KCl, 3 MgCl2, 5 EGTA and 10 HEPES, adjusted to pH 7.3 with KOH; the external solution (EXT) contained (in mM): 150 NaCl, 5 KCl, 3 MgCl2, 1 CaCl2 and 10 HEPES, adjusted to pH 7.4 with NaOH. In inside-out experiments the pipette solution contained the EXT solution and the external solution was INT. In experiments with PIP2 and TASK channels, Mg was omitted in the INT solution (Huang et al., 1998). Recordings were performed at room temperature using a RK400 patch–clamp amplifier (Bio-Logic). Data were analyzed using pClamp software. All currents were measured at 0 mV. All results are expressed as mean ± SEM, with n indicating the number of cells tested. Student’s t-tests and one-way ANOVA combined with a Student–Newman–Keuls post hoc tests were used to compare the different values, and were considered significant at P < 0.05.
Reagents
All reagents were obtained from Sigma except (RS)-3,5-dihydroxyphenylglycine (Tocris), pertussis toxin (Calbiochem), PIP2 antibodies (Molecular Probes), PKC peptide inhibitor (fragment 19–36), SAG, SLG, DOG, RHC80267, diPGP and diOGP (Biomol). All reagents were dissolved in appropriate solvents (H2O, ethanol or DMSO), with DMSO and ethanol never exceeding 1% of final solutions. Aqueous solution of SAG, SLG, DOG, diPCP, diOGP, PIP2 and RHC80267 were sonicated on ice for 30 min before use.
Acknowledgments
Acknowledgements
We thank Drs M.Borsotto, N.Malouk, R.Mengual, B.Antonny, S.Paris, C.Frelin and P.Vignes for helpful discussions. We thank N.Leroudier and M.Jodar for excellent technical assistance. We are grateful to Dr E.Honoré for help with excised patch–clamp recordings and insightful discussions. This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Institut Paul Hamel, the Association Française contre les Myopathies (AFM) and the Conseil Régional PACA.
References
- Aiba A., Kano,M., Chen,C., Stanton,M.E., Fox,G.D., Herrup,K., Zwingman,T.A. and Tonegawa,S. (1994) Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell, 79, 377–388. [PubMed] [Google Scholar]
- Ango F., Prezeau,L., Muller,T., Tu,J.C., Xiao,B., Worley,P.F., Pin,J.P., Bockaert,J. and Fagni,L. (2001) Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature, 411, 962–965. [DOI] [PubMed] [Google Scholar]
- Anwyl R. (1999) Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res. Brain Res. Rev., 29, 83–120. [DOI] [PubMed] [Google Scholar]
- Blondeau N., Lauritzen,I., Widmann,C., Lazdunski,M. and Heurteaux,C. (2002) A potent protective role of lysophospholipids against global cerebral ischemia and glutamate excitotoxicity in neuronal cultures. J. Cereb. Blood Flow Metab., 22, 821–834. [DOI] [PubMed] [Google Scholar]
- Bordi F. and Ugolini,A. (1999) Group I metabotropic glutamate receptors: implications for brain diseases. Prog. Neurobiol., 59, 55–79. [DOI] [PubMed] [Google Scholar]
- Bowlby M.R. and Levitan,I.B. (1995) Block of cloned voltage-gated potassium channels by the second messenger diacylglycerol independent of protein kinase C. J. Neurophysiol., 73, 2221–2229. [DOI] [PubMed] [Google Scholar]
- Brickley S.G., Revilla,V., Cull-Candy,S.G., Wisden,W. and Farrant,M. (2001) Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature, 409, 88–92. [DOI] [PubMed] [Google Scholar]
- Buckler K.J., Williams,B.A. and Honore,E. (2000) An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J. Physiol., 525, 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpak S., Gahwiler,B.H., Do,K.Q. and Knopfel,T. (1990) Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature, 347, 765–767. [DOI] [PubMed] [Google Scholar]
- Choi D.W. (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron, 1, 623–634. [DOI] [PubMed] [Google Scholar]
- Chuang H.H., Prescott,E.D., Kong,H., Shields,S., Jordt,S.E., Basbaum,A.I., Chao,M.V. and Julius,D. (2001) Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature, 411, 957–962. [DOI] [PubMed] [Google Scholar]
- Conn P.J. and Pin,J.P. (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol., 37, 205–237. [DOI] [PubMed] [Google Scholar]
- Conquet F. et al. (1994) Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature, 372, 237–243. [DOI] [PubMed] [Google Scholar]
- Czirjak G. and Enyedi,P. (2002) Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J. Biol. Chem., 277, 5426–5432. [DOI] [PubMed] [Google Scholar]
- Czirjak G., Petheo,G.L., Spat,A. and Enyedi,P. (2001) Inhibition of TASK-1 potassium channel by phospholipase C. Am. J. Physiol. Cell Physiol., 281, C700–C708. [DOI] [PubMed] [Google Scholar]
- Duprat F., Lesage,F., Fink,M., Reyes,R., Heurteaux,C. and Lazdunski,M. (1997) TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J., 16, 5464–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F., Lesage,F., Patel,A.J., Fink,M., Romey,G. and Lazdunski,M. (2000) The neuroprotective agent riluzole activates the two P domain K+ channels TREK-1 and TRAAK. Mol. Pharmacol., 57, 906–912. [PubMed] [Google Scholar]
- Enyeart J.J., Xu,L., Danthi,S. and Enyeart,J.A. (2002) An ACTH- and ATP-regulated background K+ channel in adrenocortical cells is TREK-1. J. Biol. Chem., 277, 49186–49199. [DOI] [PubMed] [Google Scholar]
- Fink M., Duprat,F., Lesage,F., Reyes,R., Romey,G., Heurteaux,C. and Lazdunski,M. (1996) Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J., 15, 6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Fink M., Lesage,F., Duprat,F., Heurteaux,C., Reyes,R., Fosset,M. and Lazdunski,M. (1998) A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J., 17, 3297–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum S.R. and Miller,R.J. (1992) Metabotropic glutamate receptors mediate excitatory transmission in the nucleus of the solitary tract. J. Neurosci., 12, 2251–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerineau N.C., Gahwiler,B.H. and Gerber,U. (1994) Reduction of resting K+ current by metabotropic glutamate and muscarinic receptors in rat CA3 cells: mediation by G-proteins. J. Physiol., 474, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Truell,J., Gnatenco,C. and Kim,D. (2002) Characterization of four types of background potassium channels in rat cerebellar granule neurons. J. Physiol., 542, 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbeln J.R. (1998) Fish consumption and major depression. Lancet, 351, 1213. [DOI] [PubMed] [Google Scholar]
- Hilgemann D.W., Feng,S. and Nasuhoglu,C. (2001) The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE, 2001, RE19. [DOI] [PubMed] [Google Scholar]
- Hirata M., Yanaga,F., Koga,T., Ogasawara,T., Watanabe,Y. and Ozaki,S. (1990) Stereospecific recognition of inositol 1,4,5-trisphosphate analogs by the phosphatase, kinase and binding proteins. J. Biol. Chem., 265, 8404–8407. [PubMed] [Google Scholar]
- Hofmann T., Obukhov,A.G., Schaefer,M., Harteneck,C., Gudermann,T. and Schultz,G. (1999) Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature, 397, 259–263. [DOI] [PubMed] [Google Scholar]
- Huang C.L., Feng,S. and Hilgemann,D.W. (1998) Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature, 391, 803–806. [DOI] [PubMed] [Google Scholar]
- Kim Y., Bang,H. and Kim,D. (2000) TASK-3, a new member of the tandem pore K+ channel family. J. Biol. Chem., 275, 9340–9347. [DOI] [PubMed] [Google Scholar]
- Kiselyov K. and Muallem,S. (1999) Fatty acids, diacylglycerol, Ins(1,4,5)P3 receptors and Ca2+ influx. Trends Neurosci., 22, 334–337. [DOI] [PubMed] [Google Scholar]
- Klein J., Iovino,M., Vakil,M., Shinozaki,H. and Loffelholz,K. (1997) Ontogenetic and pharmacological studies on metabotropic glutamate receptors coupled to phospholipase D activation. Neuropharmacology, 36, 305–311. [DOI] [PubMed] [Google Scholar]
- Lauritzen I., Blondeau,N., Heurteaux,C., Widmann,C., Romey,G. and Lazdunski,M. (2000) Polyunsaturated fatty acids are potent neuroprotectors. EMBO J., 19, 1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen I., Zanzouri,M., Honore,E., Duprat,F., Ehrengruber,M.U., Lazdunski,M. and Patel,A.J. (2003) K+-dependent cerebellar granule neuron apoptosis. Role of leak K+ channels. J. Biol. Chem., 278, 32068–32076. [DOI] [PubMed] [Google Scholar]
- Lesage F. and Lazdunski,M. (2000) Molecular and functional properties of two-pore-domain potassium channels. Am. J. Physiol., 279, F793–F801. [DOI] [PubMed] [Google Scholar]
- Lesage F., Guillemare,E., Fink,M., Duprat,F., Lazdunski,M., Romey,G. and Barhanin,J. (1996) TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J., 15, 1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Lesage F., Terrenoire,C., Romey,G. and Lazdunski,M. (2000) Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids and Gs, Gi and Gq protein-coupled receptors. J. Biol. Chem., 275, 28398–28405. [DOI] [PubMed] [Google Scholar]
- Maingret F., Patel, AJ., Lazdunski,M. and Honore,E. (2001) The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J., 20, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D.A. and von Krosigk,M. (1992) Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc. Natl Acad. Sci. USA, 89, 2774–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J.A., Barratt,L., Southan,A.P., Page,K.M., Fyffe,R.E., Robertson,B. and Mathie,A. (2000) A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc. Natl Acad. Sci. USA, 97, 3614–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S., Catt,K.J. and Balla,T. (1995) A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc. Natl Acad. Sci. USA, 92, 5317–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Bruno,V., Copani,A., Casabona,G. and Knopfel,T. (1996) Metabotropic glutamate receptors: a new target for the therapy of neurodegenerative disorders? Trends Neurosci., 19, 267–271. [DOI] [PubMed] [Google Scholar]
- Nicoll R.A., Malenka,R.C. and Kauer,J.A. (1990) Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol. Rev., 70, 513–565. [DOI] [PubMed] [Google Scholar]
- Patel A.J. and Honore,E. (2001a) Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci., 24, 339–346. [DOI] [PubMed] [Google Scholar]
- Patel A.J. and Honore,E. (2001b) Anesthetic-sensitive 2P domain K+ channels. Anesthesiology, 95, 1013–1021. [DOI] [PubMed] [Google Scholar]
- Patel A.J., Honore,E., Maingret,F., Lesage,F., Fink,M., Duprat,F. and Lazdunski,M. (1998) A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J., 17, 4283–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.J., Honore,E., Lesage,F., Fink,M., Romey,G. and Lazdunski,M. (1999) Inhalational anesthetics activate two-pore-domain background K+ channels. Nat. Neurosci., 2, 422–426. [DOI] [PubMed] [Google Scholar]
- Pin J.P. and Duvoisin,R. (1995) The metabotropic glutamate receptors: structure and functions. Neuropharmacology, 34, 1–26. [DOI] [PubMed] [Google Scholar]
- Runnels L.W., Yue,L. and Clapham,D.E. (2002) The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat. Cell Biol., 4, 329–336. [DOI] [PubMed] [Google Scholar]
- Sladeczek F., Pin,J.P., Recasens,M., Bockaert,J. and Weiss,S. (1985) Glutamate stimulates inositol phosphate formation in striatal neurones. Nature, 317, 717–719. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Ito,I. and Hirono,C. (1987) A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature, 325, 531–533. [DOI] [PubMed] [Google Scholar]
- Suh B.C. and Hille,B. (2002) Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron, 35, 507–520. [DOI] [PubMed] [Google Scholar]
- Talley E.M., Lei,Q., Sirois,J.E. and Bayliss,D.A. (2000) TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron, 25, 399–410. [DOI] [PubMed] [Google Scholar]
- Talley E.M., Solorzano,G., Lei,Q., Kim,D. and Bayliss,D.A. (2001) CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J. Neurosci., 21, 7491–7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks A.L., Cooke,A.M., Potter,B.V. and Nahorski,S.R. (1987) Stereospecific recognition sites for [3H]inositol(1,4,5)-triphosphate in particulate preparations of rat cerebellum. Biochem. Biophys. Res. Commun., 146, 1071–1078. [DOI] [PubMed] [Google Scholar]
- Wu L., Bauer,C.S., Zhen,X.G., Xie,C. and Yang,J. (2002) Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature, 419, 947–952. [DOI] [PubMed] [Google Scholar]