Abstract
Kinetochore microtubules (kMts) are a subset of spindle microtubules that bind directly to the kinetochore to form the kinetochore fiber (K-fiber). The K-fiber in turn interacts with the kinetochore to produce chromosome motion toward the attached spindle pole. We have examined K-fiber maturation in PtK1 cells using same-cell video light microscopy/serial section EM. During congression, the kinetochore moving away from its spindle pole (i.e., the trailing kinetochore) and its leading, poleward moving sister both have variable numbers of kMts, but the trailing kinetochore always has at least twice as many kMts as the leading kinetochore. A comparison of Mt numbers on sister kinetochores of congressing chromosomes with their direction of motion, as well as distance from their associated spindle poles, reveals that the direction of motion is not determined by kMt number or total kMt length. The same result was observed for oscillating metaphase chromosomes. These data demonstrate that the tendency of a kinetochore to move poleward is not positively correlated with the kMt number. At late prometaphase, the average number of Mts on fully congressed kinetochores is 19.7 ± 6.7 (n = 94), at late metaphase 24.3 ± 4.9 (n = 62), and at early anaphase 27.8 ± 6.3 (n = 65). Differences between these distributions are statistically significant. The increased kMt number during early anaphase, relative to late metaphase, reflects the increased kMt stability at anaphase onset. Treatment of late metaphase cells with 1 μM taxol inhibits anaphase onset, but produces the same kMt distribution as in early anaphase: 28.7 ± 7.4 (n = 54). Thus, a full complement of kMts is not sufficient to induce anaphase onset. We also measured the time course for kMt acquisition and determined an initial rate of 1.9 kMts/min. This rate accelerates up to 10-fold during the course of K-fiber maturation, suggesting an increased concentration of Mt plus ends in the vicinity of the kinetochore at late metaphase and/or cooperativity for kMt acquisition.
Proper chromosome segregation during mitosis is achieved via a complex and variable series of movements that include the rapid poleward translation of monoorienting chromosomes, oscillation of monooriented chromosomes toward and away from their attached spindle pole, bipolar attachment followed by congression to the spindle equator, oscillation of congressed chromosomes around the spindle equator, and poleward migration of sister chromatids during anaphase (reviewed in Skibbens et al., 1993; Rieder and Salmon, 1994). A wealth of evidence demonstrates that the forces producing these movements are principally derived from different types of interactions between chromosomes and spindle microtubules (Mts)1 (e.g., reviews by Rieder and Salmon, 1994; Desai and Mitchison, 1995; Inoue and Salmon, 1995). Thus, the rapid movement observed upon initial attachment is generated from interactions between the corona of the attaching kinetochore and the lateral surface of the Mts (Rieder and Alexander, 1990). The subsequent slower poleward movements (P motion) of chromosomes are generated primarily by interactions between the kinetochore and the plus ends of kinetochore microtubules (kMts) (Gorbsky et al., 1988; Nicklas, 1989; Mitchison and Salmon 1992; Inoue and Salmon, 1995; and for an alternative view, see Pickett-Heaps et al., 1996). Finally, movements of chromosomes away from their attached spindle pole (AP motion) are caused by an antagonistic pull on one of the kinetochores and non-kMts exerting an ejection force along the full length of chromosomes (reviewed in Rieder and Salmon, 1994; Khodjakov and Rieder, 1996).
After initial attachment, kinetochore P motion is accompanied by kMt disassembly, while AP motion is accompanied by kMt assembly (reviewed in Hyman and Mitchison, 1992), with addition and removal of Mt subunits during assembly and disassembly occurring primarily at the kinetochore (reviewed in Mitchison and Salmon, 1992). For this reason, kinetochore–Mt interactions during P and AP motion are distinct, differing both in direction of motion along the Mt lattice and in the dynamic instability state of kMts. At all stages of mitosis, between initial attachment and mid-anaphase, kinetochores are capable of abruptly switching between P and AP motion (reviewed in Skibbens et al., 1993; Khodjakov and Rieder, 1996). As a result, attached kinetochores on both monooriented and bioriented chromosomes frequently oscillate between stretches of P and AP movement without affecting a net change in chromosome position. Oscillations continue even during congression and anaphase, but in these stages of mitosis, net chromosome displacement occurs because individual kinetochores are biased to spend a greater percentage of time moving in one particular direction (Skibbens et al., 1993).
One of the unresolved issues of chromosome motion is the molecular mechanism for biasing kinetochore movement during congression. Traction fiber models postulate that the strength of poleward force acting on the kinetochore is proportional to the length of its kinetochore fiber (K-fiber; e.g., Hays et al., 1982; Pickett-Heaps, 1986; Fuge, 1989). In support of this model, Hays and Salmon (1990) found in meiotic grasshopper spermatocytes that when one kinetochore of a fully congressed chromosome is partially destroyed by microbeam irradiation, the chromosome moved toward the pole attached to the undamaged sister, with the final ratio of distances from the two poles being inversely proportional to the ratio of the number of kMts on each kinetochore. The authors interpreted this data to reveal that the poleward force generated at each kinetochore is proportional to the length of the K-fiber times the number of kMts. On the other hand, Hyman and Mitchison (1991a,b) argue from in vitro results that the tendency of a kinetochore to move P decreases with the acquisition of kMts. In their model, a minus-end motor that is active during initial attachment is turned off as the attached kinetochore of a monooriented chromosome acquires more kMts. When that chromosome becomes bioriented, the previously unattached sister kinetochore is initially more strongly biased toward P motion than its sister because of a lower kMt number. This results in net displacement toward the spindle equator, and hence congression. In contrast to both of these models, Rieder and Salmon (1994; see also Khodjakov and Rieder, 1996) argue that tension created by polar ejection forces, as well as the antagonistic pull of the sister kinetochore, causes a kinetochore to switch out of the P-moving state. In their model, the polar ejection forces provide sufficient information about location on the spindle to explain congression, and the number of kMts in each K-fiber is relatively unimportant.
The kinetochore has also been implicated in control of the cell cycle. In many cells, transition into anaphase is inhibited by a cell cycle checkpoint (Hartwell and Weinert, 1989) that monitors unattached kinetochores (Rieder et al., 1994, 1995). This checkpoint also appears to be sensitive to treatments that disrupt spindle Mt structure or interfere with Mt dynamic instability (e.g., Jordan et al., 1992; Wendell et al., 1993; Rieder et al., 1994). For example, treatment of HeLa cells with concentrations of vinblastine that inhibit Mt dynamic instability without promoting Mt disassembly blocks entry into anaphase without major disruption of spindle organization (Wendell et al., 1993). One of the few structural differences detected in the spindles of treated cells was that K-fibers had 30% fewer kMts. These results suggest that a full complement of kMts might be required to pass the checkpoint, and that this may be the mechanism by which damping dynamic instability inhibits anaphase onset (Rieder et al., 1994).
Although the kMt number is potentially an important factor in defining the direction of kinetochore (i.e., chromosome) motion and anaphase onset, there is little reliable data on how kMt number changes with the duration of kinetochore attachment, direction of motion, or the stage of mitosis. In animal cells, reliable kMt counts have been determined only on metaphase or anaphase chromosomes (e.g., Brinkley and Cartwright, 1971; Roos, 1973; McIntosh et al., 1975; Rieder, 1981a ; Cassimeris et al., 1990; McDonald et al., 1992), and no one attempted to correlate the direction of chromosome motion with Mt numbers on sister kinetochores. In this report, we present a comprehensive examination of K-fiber maturation during the course of mitosis in PtK1 cells. Same-cell correlative video light microscopy/serial-section EM was used to correlate the number of kMts per kinetochore with the direction of chromosome motion, length of the K-fiber, duration that kinetochores were attached to the spindle, and the stage of mitosis. We also examined metaphase cells that were inhibited from entering anaphase by treatment with taxol. The results demonstrate that the P motion of the leading kinetochore on a congressing chromosome can be induced and sustained by only one or several Mts, even when the trailing kinetochore is attached to its pole by many more Mts. Therefore, the tendency of a kinetochore to move P is not positively correlated with kMt numbers. We also found that a full complement of kMts is not sufficient to overcome the checkpoint controlling the metaphase/ anaphase transition.
Materials and Methods
Video-enhanced Light Microscopy
Stock cultures of PtK1 cells (2N = 11) were maintained in Hepes-buffered L15 media and grown on glass coverslips, as described by Rieder et al. (1994). For light microscopic observation, coverslips were mounted with VALAP, in culture media, on perfusion chambers (reviewed by Rieder and Cole, 1997). Chromosome motion in mitotic cells was recorded by time-lapse video, using differential interference contrast, on a Nikon Microphot FX (Melville, NY), with a 60× (NA = 1.4) objective, as described by Waters et al. (1993). The recording rate was 15 frames/min, and the culture was maintained at 37°C using a block heater for the specimen stage. Some cells were treated with 1 μM taxol (Calbiochem Corp., San Diego, CA) in media containing 1% DMSO. At the appropriate time, cells were rapidly fixed by perfusion with 2.5% glutaraldehyde in 0.1 M phosphate buffer. As reported by Rieder and Alexander (1990), all motion ceased within one frame after the fixative reached the cell. After fixation, low magnification frames were recorded, and the position of the filmed cell was marked on the glass coverslip with an objective scribe. Coverslips were then transferred to small chambers and processed for EM. Tracking records were plotted from measurements of the distance between a kinetochore and its associated spindle pole in successive frames of the video record, as described by Khodjakov and Rieder (1996).
Electron Microscopy
Cells were fixed for a total of 30 min in 2.5% glutaraldehyde, washed with phosphate buffer, and postfixed for 60 min with 2% OsO4. They were then treated for 1 min with 0.15% tannic acid and stained en bloc for 60 min with 1% uranyl acetate. En block staining was followed by a graded ethanol dehydration series, clearing with propylene oxide, and flat embedding in Epon (see Roos, 1973). After curing, scribe marks were transferred to the Epon, the glass coverslips were removed by etching, and the resulting preparations were trimmed and glued onto an Epon block for sectioning (Rieder, 1981b ). Silver-gray serial thin sections (∼75 nm thick) were collected on Formvar-coated slot grids and subsequently stained with uranyl acetate and lead citrate.
Consecutive serial sections were viewed on an electron microscope (model 910; Carl Zeiss, Inc., Thornwood, NY), using an accelerating voltage of 80 kV, an LaB6 filament, and the Köhler illumination system. Images of metaphase, anaphase, and taxol-treated cells were recorded at ×5,000, and those of prometaphase cells at ×3,150 or ×4,000 on electron microscope film (S0163; Kodak, Rochester, NY). At these magnifications, all the spindle contained in each section could be captured on a single negative, and selected areas of the negative could be enlarged to identify kMts accurately.
Data Analysis
kMts were counted from digitally scanned micrographs of serial sections. The kinetochore region of each chromosome was scanned into a 320 × 320–pixel window using a modified Dage model 81 camera (Michigan City, IN) with an Androx ICS-400 image-processing board (Imaging Solutions Corporation, Natick, MA) in a Sun 3/260 host computer (Mountain View, CA) (Hindus, 1992). The enlargements used were obtained by fitting the camera with a 105-mm lens and an auto extension ring. The image pixel size was typically 2.7, 3.0, and 4.7 nm, respectively, for images recorded at ×5,000, ×4,000, and ×3,150. These values correspond to 13.5, 12.0, and 14.7 μm on the film. Objects of the appropriate size and shape were identified as kMts if they came within 50 nm of the outer kinetochore plate or were embedded in the corona material and had an image density at least one third the density of the brightest kMts in the same digitized image. The analysis was aided by varying image contrast and by placing images of the same kinetochore from neighboring serial sections side by side on the computer monitor (Silicon Graphics, Mountain View, CA, INDY). If the same kMt was identified in neighboring serial sections, it was only counted in one section.
From each kinetochore, kMts were counted at least twice, on different days, by the same person, and the average value of the counting trials was recorded. If the counting trials differed by more than 20%, the number was counted again, and the best fit to all trials was recorded. The counting error was assessed from the variation between duplicate (or in some cases triplicate) counting trials in the following way. For each kinetochore, the variation between multiple counting trials was used to calculate a “counting standard deviation” that was then divided by the reported kMt value. The latter operation normalized values so that counting standard deviations from different kinetochores could be averaged. The average counting standard deviation of 53 kinetochores, from three different cells, was 0.08. Since the normalized value is a fractional value (i.e., a ratio between the counting standard deviation and kMt number), we concluded that the counting error was 8%, or ±2.0 kMts for kinetochores with 25 kMts. To assess possible bias introduced by having one person make all of the counts, eight other people were asked to count the number of kMts on four metaphase kinetochores using the stated criteria. On average, the kMt number was the same as when a single person made the counts, but the estimated counting error was slightly higher (12%).
Distances between the spindle poles and kinetochores of monooriented or congressing chromosomes were measured in three dimensions, using the Sterecon software package for serial section reconstruction and quantitative measurements (Marko and Leith, 1996). Electron micrographs of the serial section series were first examined on a light box to determine which sections contained the midpoints of the spindle poles and the kinetochores. The corresponding micrographs were then digitized (pixel size = 20–30 nm) in sequence with enough of the intervening sections to enable alignment in Sterecon. The depth dimension of the resulting image stack was adjusted for the excluded sections, using an assumed section thickness of 75 nm. Coordinates for the centers of poles and kinetochores were obtained within the resulting Sterecon reconstruction and were used to calculate three-dimensional (3D) distance vectors using the standard distance formula.
For statistical analyses, the relevant data were entered into Quattro Pro version 6.0 (Novell, Inc., Orem, Utah), and the functions for computing averages, standard deviation, Student's t test, z test, and regression analysis were used. For comparisons of prometaphase, metaphase, anaphase, and taxol distributions, the z test was appropriate because of the relatively large sample sizes (n > 30). For comparisons of kMt numbers on sister kinetochores of bioriented chromosomes, the t-test was used because of the small sample sizes. Analyses involving the time course of kMt acquisition were aided by Mathematica 2.2 (Wolfram Research, Inc., Champaign, IL).
Equations
Initially we modeled K-fiber maturation as a simple association/dissociation reaction:
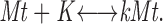 |
1 |
For this model, the rate at which kinetochores acquire kMts is
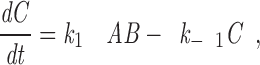 |
2 |
where A is the concentration of free Mt plus ends, B is the number of unoccupied sites on a kinetochore, C is the number of occupied sites (i.e., the number of kMts), and k 1 and k−1 are the association and dissociation rate constants. Since the growth of free Mts is continually initiated from the spindle pole in large excess of the number of kMts, to a first approximation, the concentration of free Mt plus ends is independent of the course of the reaction. In addition, the number of unoccupied binding sites, B, can be determined from the number of occupied sites, C, and the total binding capacity (number of kMts bound at saturation), S, which is a constant. Substituting these identities and rearranging, the rate equation becomes
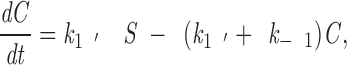 |
3 |
where
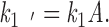 |
4 |
The solution to Eq. 3 is
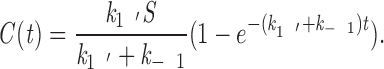 |
5 |
Since there are no kMts on the kinetochore until it becomes attached, C is 0 at time zero, and the initial rate of this pseudo–first order reaction is linear:
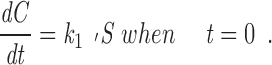 |
6 |
At equilibrium, the forward and reverse reactions must be balanced and, after rearrangement, the rate equation becomes
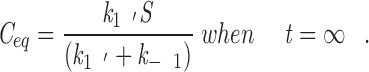 |
7 |
For the more complex model, where the association rate increases with time, we estimated the variation of k 1 with time empirically:
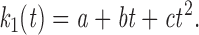 |
8 |
By fitting our data to this function, we found that a = 0.05735, b = 0, and c = 0.00013. Modification of Eq. 3 to include the time dependence of k 1 results in
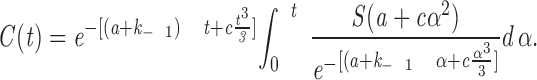 |
9 |
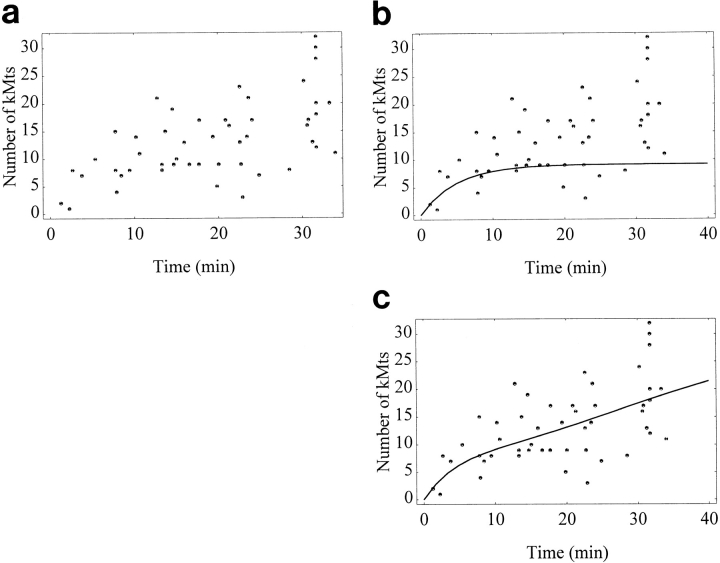
Substituting the above constants a and c, as well as the previously determined saturation value and dissociation constant, results in the curve illustrated in Fig. 6 c.
Figure 6.
Time course for kMt acquisition. (a) Raw data. The length of time that monooriented, bioriented, and congressing chromosomes were attached to the spindle was determined from video records, as described in the text. (b) Curve predicted by a simple association/dissociation model (see Eq. 5). The association rate constant k 1 is estimated to be 0.053 from the earliest time points (see Eq. 4 a and text), the kMt value at saturation S is approximated as 35 kMts, and the dissociation rate constant k −1 is set at 0.15 kMt/min, based on a kMt half-life of 4.7 min at 37°C (Zhai et al., 1995). The resulting curve levels off at 9.2 kMts, far fewer than the 24 kMts observed at metaphase (Table IV). (c) Curve predicted by a model where k 1 increases with time (see Eq. 9). This curve is consistent with observations of both the initial rate of kMt acquisition and the kMt number at metaphase.
Results
A total of 36 mitotic cells were filmed, fixed at the appropriate time, and processed for subsequent serial section analysis. Mts were scored as kMts if they came within 50 nm of the kinetochore outer plate or were embedded into the corona material and had at least one third the density of the brightest kMts in the same digitized image (Fig. 1). All counts were determined at least in duplicate, as described in Materials and Methods, and from the multiple counting trials of 53 different kinetochores, the counting error was estimated to be 8%, or ±2.0 per 25 kMts.
Figure 1.
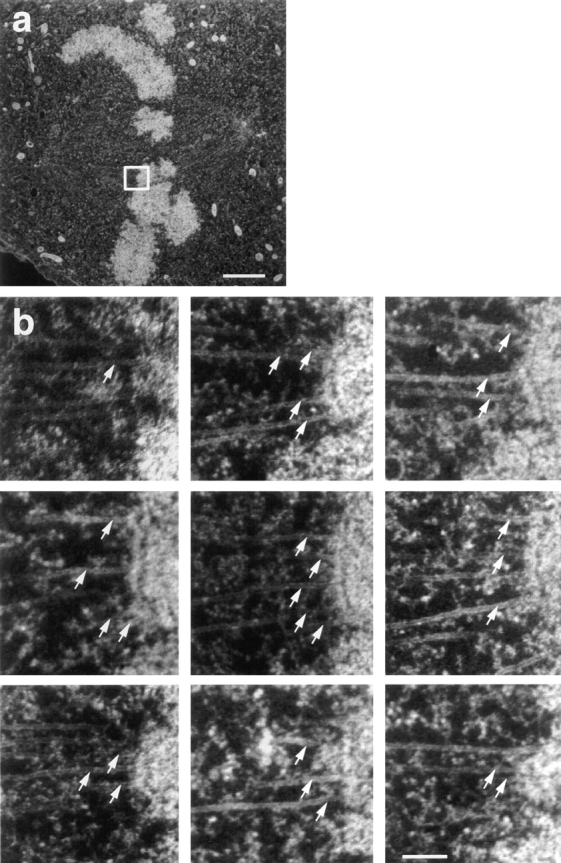
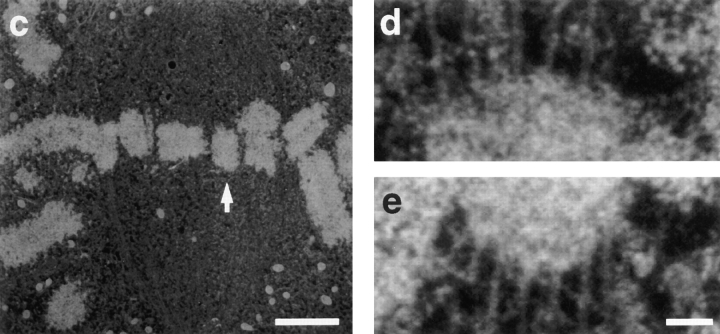
Illustration of our method for counting kMts from serial section electron micrographs. (a) Full area of one electron micrograph from a cell. Electron micrographs were taken at a magnification sufficiently low to record all of the mitotic spindle, and were then enlarged for counting kMts. The white box indicates one kinetochore and its associated microtubules to be counted in consecutive serial sections. (b) The outer plate of the kinetochore indicated in a could be identified in nine serial sections. Associated microtubules were counted as kMts when they were visible within 50 nm from the outer kinetochore plate or were embedded in the corona material. A total of 28 kMts were counted on this kinetochore (arrows). Bars: (a) 2 μm; (b) 0.2 μm.
Mt Numbers on Sister Kinetochores of Congressing Chromosomes
As a monooriented chromosome becomes bioriented, the newly attaching kinetochore is expected to have fewer Mts than its sister because the latter has been attached to the spindle for a longer time. However, the extent of this disparity has not been previously investigated, and little is known concerning how many Mts are needed to initiate congression (i.e., P motion of the newly attaching kinetochore) or the rate at which kinetochores acquire their full complement of kMts. We filmed late prometaphase cells (i.e., cells containing one to four monooriented chromosomes) until one of the monooriented chromosomes exhibited the in-plane rotation characteristic of biorientation and began moving toward the metaphase plate with the centromere region leading (e.g., Fig. 2, a and b). When this motion was well established, cells were rapidly fixed and processed for EM. Chromosomes tracked in vivo (see Materials and Methods) were readily identified from low magnification electron micrographs (Fig. 3 a), and kMts were counted from enlargements of the kinetochore region, as illustrated in Fig. 1 b.
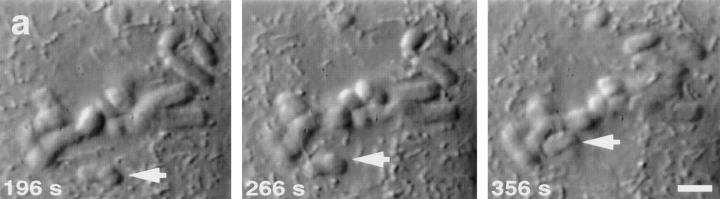
Figure 2.
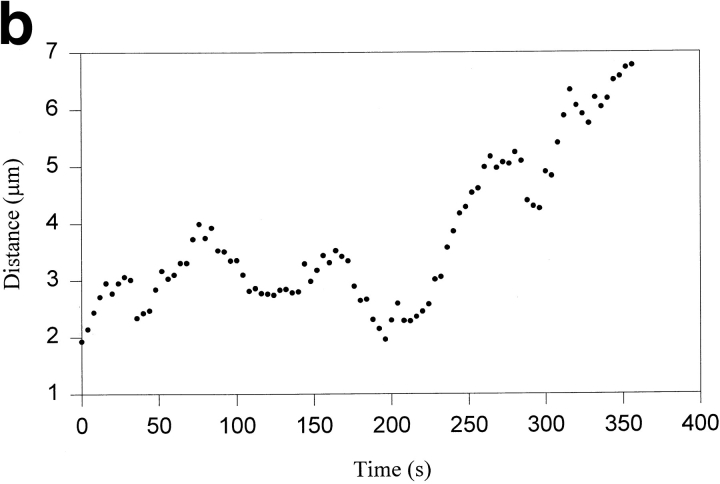
Tracking of a congressing chromosome as identified by differential interference contrast (DIC) video-enhanced light microscopy. (a) Selected frames from the DIC-video record. The congressing chromosome is indicated by the white arrow, and the time after initiation of video tracking is given in the lower left corner of each frame. (b) Tracking record of the AP-moving kinetochore on the chromosome noted in a as it initiates congression and moves to the spindle equator. From the start of filming until ∼196 s, the chromosome is monooriented and oscillates without a net change in distance from the spindle pole. At ∼196 s, the chromosome acquires bipolar attachment and initiates congression. During congression, the AP-moving kinetochore exhibits a brief reversal at ∼290 s and a pause at ∼330 s. Fixation is at 356 s. Bar, 5 μm.
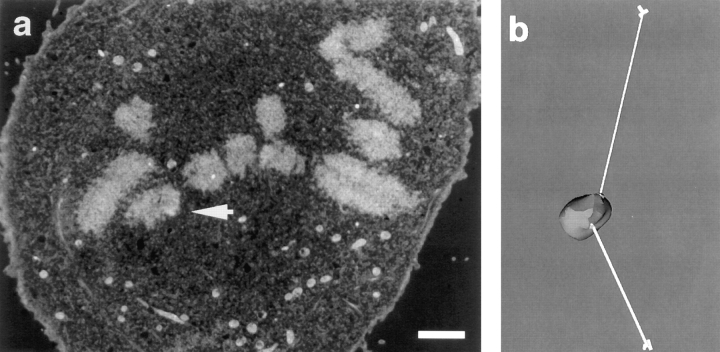
Figure 3.
Serial section electron microscopic analysis of the congressing chromosome from Fig. 2 to determine kMt numbers and distance from spindle poles. (a) An electron micrograph from a serial section series through the cell. White arrow indicates the AP-moving kinetochore of the congressing chromosome. (b) Measurement of the distances between sister kinetochores and their associated spindle poles. The congressing chromosome was reconstructed in 3D from serial sections, as described in the text. Sister kinetochores are indicated by the light regions at the site where K-fibers (white tubes) attach to the chromosome (gray). The centrioles are schematically represented by cylinders. Distances between kinetochores and the spindle poles were determined by measuring the K-fiber length in 3D. Bar, 2 μm.
The number of kMts found on sister kinetochores of five congressing chromosomes are listed in Table I. For all five determinations, at least twice as many kMts were attached to the AP-moving kinetochore, and in most cases, the disparity is much greater. To compare congressing chromosomes with fully congressed chromosomes, we defined a disparity ratio as the number of Mts attached to the sister kinetochore with the most Mts divided by the number of Mts attached to the sister with the least Mts. For congressing chromosomes, this ratio was always the number of Mts on the AP-moving kinetochore divided by the number on the P kinetochore. A comparison between disparity ratios of congressing and fully congressed chromosomes in the same prometaphase cells is given in Table II. (Fully congressed chromosomes were defined as those positioned at the metaphase plate.) Clearly, the number of kMts is more balanced on sister kinetochores of fully congressed chromosomes than it is on sister kinetochores of congressing chromosomes. Only a few of the former have disparity ratios greater than 2, and in some cases, the film record shows these to be newly congressed. Table II also shows that the disparity between sisters drops further as cells progress into metaphase, where the maximum ratio observed was only ∼1.5.
Table I.
Number of kMts and Distances from Spindle Poles for Congressing Chromosomes
| Congressing chromosome | No. of kMts | Distance from spindle pole | Predicted relative force | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell No. | AP | P | AP | P | AP | P | ||||||
| μm | (NL)* | |||||||||||
| 1 | 16 | 8 | 3.9 | 8.5 | 62.4 | 68 | ||||||
| 2 | 27 | 8 | 6.5 | 9.4 | 175.5 | 75.2 | ||||||
| 3 | 26 | 1 | 5.8 | 18.3 | 150.8 | 18.3 | ||||||
| 4 | 11 | 2 | 5.8 | 10.0 | 63.8 | 20.0 | ||||||
| 5‡ | 32 | 7 | 10.9 | 7.8 | 348.8 | 54.6 | ||||||
After the formulation of Hays and Salmon (1990). See text for details.
Congressing chromosome from cell 5 has traveled beyond the metaphase plate.
Table II.
Disparity Ratios for Congressing, Late Prometaphase, and Late Metaphase Chromosomes
| Range of disparity ratio* | Number of sister pairs | |||||
|---|---|---|---|---|---|---|
| Congressing‡ | Fully congressed‡ | Mature metaphase§ | ||||
| 1.00–1.15 | 0 | 14 | 17 | |||
| 1.16–1.30 | 0 | 13 | 8 | |||
| 1.31–1.45 | 0 | 7 | 4 | |||
| 1.46–1.60 | 0 | 6 | 1 | |||
| 1.61–1.75 | 0 | 3 | 0 | |||
| 1.76–3.00 | 1 | 4 | 0 | |||
| >3.00 | 4 | 0 | 0 | |||
Number of kMts on the sister kinetochore containing the most kMts divided by the number on the sister containing the least.
Congressing chromosomes were identified in late prometaphase cells (cells with one to four monooriented chromosomes) by their movement towards the spindle equator. All other chromosomes that were situated at the spindle equator in these cells were considered fully congressed.
Mature metaphase chromosomes came from cells filmed for 20–21 min after the last monooriented chromosome–initiated congression.
From their data, Hays and Salmon (1990) argued that the poleward force on a kinetochore depends on the kMt number and the K-fiber length (i.e., F = cNL, where F = force, L = length of K-fiber, measured as the distance between the kinetochore and its associated spindle pole, N = number of kMts, and c is a proportionality constant). Assuming as Hays and Salmon (1990) did, that the predominate direction of motion is indicative of which sister kinetochore experiences the greater poleward force, their model predicts that the product of kMt number and K-fiber length should be greater for the P-moving kinetochore during congression (i.e., N AP L AP < N P L P). We evaluated this prediction for the P and AP kinetochores listed in Table I by measuring K-fiber lengths in 3D, as illustrated in Fig. 3 b, and multiplied by the corresponding kMt numbers. The resulting values are listed in Table I. Contrary to the prediction of Hays and Salmon's balanced force model, the product of length and number of kMts is 2.3– 8.4 times greater for the AP kinetochore in four of the five congressing chromosomes. Thus, poleward force at the kinetochore is clearly not proportional to kMt number times K-fiber length during congression in PtK1 cells, and the Hays and Salmon (1990) assumption that the sister kinetochore possessing the most Mts experiences the greater P force is not valid.
Comparing Mt Numbers on Sister Kinetochores of Oscillating Bioriented Chromosomes
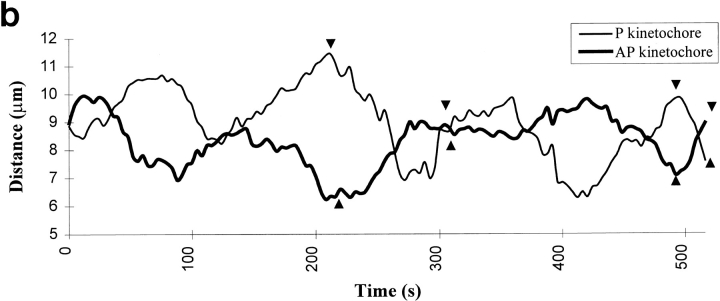
We investigated the possibility that poleward force acting on the sister kinetochores of fully congressed chromosomes is related to the kMt number by determining Mt numbers on the sister kinetochores of fully congressed but oscillating chromosomes whose motion could be defined by the video record at the time of fixation (Fig. 4). AP-moving kinetochores had 25 ± 2.7 kMts, and P-moving kinetochores had 21 ± 3.1 kMts (Table 3A). These differences are on the borderline of statistical significance (P = 0.028 in a two-tail t test), primarily because of the small sample sizes. One reason for the small sample size was that in any one optical plane, only a few of the chromosomes were oscillating to a significant extent during filming. As an alternative approach, we examined the film record of chromosomes with varying disparity in the number of kMts associated with each sister kinetochore. As seen in Table 3B, the likelihood of pronounced oscillations was not correlated with the difference in the kMt number between sister kinetochores.
Figure 4.
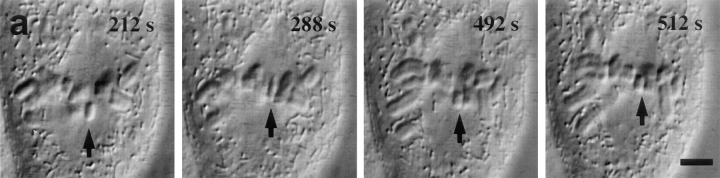
Correlating kMt numbers with the direction of motion of sister kinetochores on an oscillating metaphase chromosome. (a) Frames from the DIC video record showing the oscillating chromosome, indicated by black arrows. (b) Tracking record for sister kinetochores of the oscillating chromosome. Arrowheads correspond to frames shown in a. At fixation (512 s), one kinetochore is moving P and the other is moving AP. (c) A serial section electron micrograph of the cell. Oscillating chromosome is indicated by white arrow. (d) Electron micrograph enlarged to show the P kinetochore and its associated kMts. The kMts were counted in successive serial sections, as illustrated in Fig. 1. (e) Electron micrograph enlarged to show the AP kinetochore and its associated kMts. Bars: (a) 4 μm; (c) 2.2 μm; (d and e) 0.12 μm.
Table 3A.
Direction of Motion vs. Number of kMts on Individual Kinetochores
Includes kinetochores in transition from AP to P.
Includes kinetochores in transition from P to AP.
Difference in kMt numbers for P and AP kinetochores is statistically significant: P = 0.028 for two-tailed t test.
Table 3B.
Detectable Motion vs. Difference in Number of kMts on Sister Kinetochores
| Difference in kMts | n | Number in motion | ||
|---|---|---|---|---|
| 0–3 | 10 | 3 | ||
| 4–6 | 9 | 4 | ||
| 7–11 | 6 | 1 |
Comparing Mt Numbers on Fully Congressed Kinetochores in Prometaphase, Late Metaphase, and Early Anaphase Cells
As detailed in Table I, the number of kMts on the AP-moving kinetochore of congressing chromosomes varies from 11 to 32. Kinetochores of fully congressed prometaphase chromosomes show a similar range in their number of attached kMts (Table IV). This wide variability, as well as the observation that none of the P kinetochores on congressing chromosomes had more than eight kMts, indicates that kinetochores acquire kMts relatively slowly, and that only a few prometaphase kinetochores have a full complement of kMts. If this is true, then kinetochores in late metaphase cells should, on average, have more kMts than prometaphase kinetochores, and the distribution of kMt number should have less variability (i.e., a higher percentage of the kinetochores are expected to have a full complement of kMts). To evaluate this prediction, we determined the number of kMts on nearly all of the kinetochores from three metaphase cells that were fixed 20–21 min after the last monooriented chromosome-initiated congression. Since anaphase onset in PtK1 cells occurs at 23 min after the last monooriented chromosome initiates congression (with a standard deviation of 1 min, Rieder et al., 1994), these cells were in late metaphase at the time of fixation. The distributions of kMt numbers for prometaphase and metaphase kinetochores are compared in Fig. 5 a and Table IV. This comparison demonstrates that on average, late-metaphase kinetochores have more kMts and less variability than prometaphase kinetochores, and the difference of the mean values is statistically significant (P = 6.5 × 10−7 in a one-tail z test).
Table IV.
Variation of kMt Numbers with the Stage of Mitosis and Taxol Treatment
| Mono- oriented | Late Pro-metaphase* | Mature metaphase‡ | Early anaphase§ | Taxol (10 min)‖ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average | 11.9 | 19.7 | 24.3 | 27.8 | 28.7 | |||||
| Standard deviation | 5.9 | 6.7 | 4.9 | 6.3 | 7.4 | |||||
| Range | 3–27 | 7–34 | 11–35 | 14–44 | 12–52 | |||||
| n | 30 | 94 | 62 | 65 | 54 |
Late prometaphase chromosomes came from cells with 1–4 monooriented chromosomes.
Mature metaphase chromosomes came from cells filmed for 20–21 min after the last monooriented chromosome initiated congression.
Early anaphase chromosomes came from cells fixed 1–3 min after chromatid separation.
Metaphase cells (all chromosomes at the spindle equator) were treated for 10 min with 1.0 μM taxol before fixation.
Figure 5.
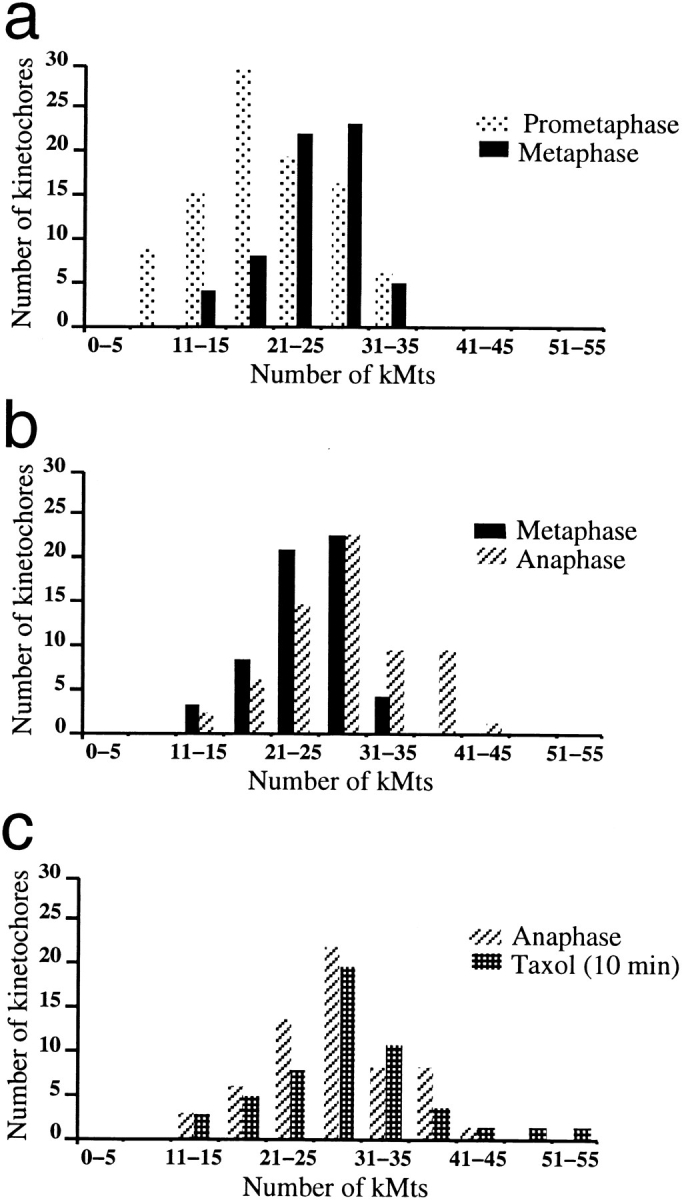
Numbers of kMts on late prometaphase, mature metaphase, early anaphase, and taxol-treated metaphase cells. The mitotic stages are defined in Table IV. (a) A comparison of kMt numbers from late prometaphase and mature metaphase kinetochores reveals that the prometaphase kinetochores are less saturated than the metaphase kinetochores. (b) A comparison of kMt numbers on late metaphase and early anaphase kinetochores reveals that mature metaphase kinetochores are less saturated than early anaphase kinetochores. (c) A comparison of kMt numbers on taxol-treated (10 min) metaphase and early anaphase kinetochores reveals no difference in the distribution.
Similarly, kinetochores from bioriented prometaphase chromosomes are expected to have more kMts than kinetochores from monooriented prometaphase chromosomes because the bioriented kinetochores on average have been attached longer and because tension between sister kinetochores stabilizes kMt attachments, at least during meiosis in grasshopper spermatocytes (Nicklas and Ward, 1994). As summarized in Table IV, kinetochores from bioriented prometaphase chromosomes have an average of 19.7 ± 6.7 kMts, while kinetochores from monooriented prometaphase kinetochores had only 11.9 ± 5.9 kMts. This difference is highly significant (P = 4.4 × 10−10 in a one-tail z test).
Even at late metaphase, the kMt number varies over a relatively large range (Table IV), indicating that some kinetochores have not acquired their full complement of kMts and/or that kinetochore size (i.e., total kMt binding capacity) is variable. In fact, kinetochore size does vary over a three- to fourfold range (McEwen, B.F., A.B. Heagle, and C.L. Rieder, manuscript in preparation), but in the present analyses we circumvent this variably by looking at the average value for all or nearly all chromosomes in each cell.
To explore the possibility that late metaphase kinetochores are subsaturated, we determined the kMt number on early anaphase kinetochores. Cells were filmed until it was certain that anaphase had commenced (1–3 min after chromatid separation), and then they were fixed for EM. The average number of kMts observed at early anaphase was 27.8 ± 6.3, or 3.5 more kMts than at metaphase (Table IV). Distributions of kMt number for late metaphase and early anaphase are compared in Fig. 5 b. Clearly, the distribution shifts toward higher kMt numbers after anaphase onset and this difference is statistically significant (P = 4.2 × 10−4 in a two-tail z test).
The Effect of Taxol Treatment on kMt Number
Moderate doses of taxol inhibit anaphase onset in PtK1 cells, presumably by preventing passage through a cell cycle checkpoint (e.g., Rieder et al., 1994). It is generally assumed that the average 23-min delay that occurs between attachment of the last kinetochore to the spindle and anaphase onset is the time required for biochemical events downstream from the checkpoint release. However, this could also represent the time required for the last attaching kinetochore to acquire the minimum complement of kMts that is needed to shut down production of the anaphase inhibitor (see Rieder et al., 1995). This would explain why HeLa cells treated with low levels of vinblastine are blocked from anaphase onset, since treated cells have 30% fewer kMts per kinetochore (Wendell et al., 1993). Similarly, it is possible that taxol exerts its inhibition by causing kMts to detach from the kinetochore.
To evaluate this hypothesis, we determined the Mt number on metaphase kinetochores after treatment for 1 h with 1.0 μM taxol. The average kMt number was 23.2 ± 7.6, which is similar to the value observed on mature metaphase kinetochores, but the standard deviation (7.6) and the maximum number of kMts observed (46) were both higher. In addition, we noticed that the spindles were abnormally short after 1 h of taxol treatment, with some kinetochores as close as 2.2 μm to the pole (data not shown). This observation is consistent with a recent report by Waters et al. (1996a) that the spindle shortens during taxol incubation because of the removal of kMt subunits at the centrosome in the absence of plus-end assembly. Since it is possible that the average kMt number has also been reduced by minus-end disassembly, we determined the number of Mts on kinetochores from metaphase cells treated for only 10 min with 1.0 μM taxol. These kinetochores had 28.7 ± 7.4 kMts (Table IV), and their Mt distribution was nearly identical to the kMt distribution found in early anaphase (Fig. 5 c). Differences between anaphase and 10-min taxol-treated cells are not statistically significant (P = 0.48 in a two-tail z test).
K-Fiber Maturation
The time course of K-fiber maturation was measured directly by filming cells from before nuclear envelope breakdown until fixation after specified lengths of time. kMts were counted on those kinetochores whose total time of attachment to the spindle could be determined unambiguously, including kinetochores from mono- and bioriented chromosomes and the congressing kinetochores reported in Table I. In most cases, initial attachment was recognized by a sudden jerk toward the spindle pole, followed by continuous P motion until the chromosome encountered an obstruction or underwent an oscillation. Frequently, chromosomes could be followed for up to 30 min after initial attachment. The data show considerable scatter in the time course (Fig. 6 a), indicating a large variability in the rate of kMt acquisition. Since the rate of kMt acquisition is expected to be proportional to the total Mt-binding capacity of a kinetochore, some of the scatter in Fig. 6 a undoubtedly arises from variations in the Mt-binding capacity between kinetochores. In addition, we observed indications that steric hindrance can affect the rate of kMt acquisition: in one prometaphase cell, several kinetochores were partially shielded from their attached spindle pole by the arms of other chromosomes, and all of these kinetochores had significantly fewer kMts than neighboring kinetochores that were on the spindle for an equivalent length of time (data not shown and not included in Fig. 6).
Based on our observations of K-fiber maturation, we determined a model to describe the process according to a simple association/dissociation reaction, such as the one illustrated by Zhai et al., (1995) (also see Eqs. 1–7 in Materials and Methods). All parameters of the model (Eq. 5) were experimentally determined. The maximum Mt binding capacity of kinetochores was estimated to be 35 from the average kinetochore size and the maximum kMt packing density (see Rieder, 1982; McEwen, B.F., A.B. Heagle, and C.L. Rieder, manuscript in preparation). The dissociation rate was set at 0.15 (min)−1, based on the 4.7-min half-life of kMts in PtK1 cells at 37°C (Zhai et al., 1995). The association rate, k 1, was estimated to be 0.053 (min)−1 from the initial velocity of the reaction, 1.9 kMt/min. The latter was computed as specified in Eq. 6, using the first five time points from Fig. 6 a (i.e., the first 6 min of the reaction). These values were substituted into Eq. 5 to compute expected kMt values over the first 40 min. The resulting curve is shown in Fig. 6 b, overlaying the data points of the measured time course. The computed equilibrium value (i.e., the plateau value of the overlay in Fig. 6 b) is 9.2 kMts, far fewer than the average value of 24 kMts per kinetochore observed at late metaphase (Table IV). To achieve an equilibrium value of 24 kMts/kinetochore, the initial velocity needs to be 16.7 kMt/min, far faster than the observed initial rate of 1.9 kMt/min, unless the dissociation constant k −1, is lowered to 10−5 min−1. However, the latter corresponds to a kMt half-life of more than 3 h!
Since the time course data cannot be described by the simple association/dissociation model, we explored the possibility that the kMt acquisition rate increases during the course of the reaction (see Eqs. 8 and 9 in Materials and Methods). This could happen if the concentration of free Mt plus ends increases during the course of the reaction (see Eq. 4 a), and/or if there is cooperativity in kMt binding. The resulting curve, shown as an overlay on the data points in Fig. 6 c, satisfies the initial slow rate of kMt acquisition and approaches the equilibrium level observed at metaphase.
Discussion
Measurements of Kinetochore Microtubule Number
Assessment of the variation between duplicate and triplicate kMt counts established that our average counting error is 8%. Since this is much less than the observed variation in kMt numbers (e.g., Table IV), counting error is not a limiting factor in our analysis. A variation of 12% was determined when eight different people counted, which indicates that the relative accuracy of our determinations is higher than the absolute accuracy, despite efforts to carefully define which Mts will be counted as kMts. Nevertheless, our results for kMt numbers at metaphase agree well with previous studies of PtK1 kinetochores from several different laboratories (Table V), using such widely different approaches as serial longitudinal sections (Brinkley and Cartwright, 1971; Roos, 1973; McIntosh et al., 1975; Cassimeris et al., 1990), serial axial sections (Rieder, 1981a ; McDonald et al., 1992), cold treatment to depolymerize non-kMts (Rieder, 1981a ), and a 3D reconstruction via electron tomography (McEwen et al., 1995). This agreement with previously published results substantiates the validity of our methodology. It is also noteworthy that we counted more kinetochores to determine the metaphase distribution of kMt number than all of the previous studies combined.
Table V.
Previous Determinations of kMt Number on Metaphase PtK1 Kinetochores
| Study | Average | Standard deviation | Range | n | ||||
|---|---|---|---|---|---|---|---|---|
| Brinkley and Cartwright (1971) | 25 | N.A. | 21–30 | 10 | ||||
| Roos (1973) | 26 | N.A. | 16–40 | 8 | ||||
| McIntosh et al. (1975) | 31 | N.A. | 21–41 | 8 | ||||
| Rieder (1981) | 29 | N.A. | 20–45 | 10 | ||||
| Cassimeris et al. (1990) | 24 | 5.0 | 19–29 | N.A. | ||||
| McDonald et al. (1992) | 25.5 | 5.2 | N.A. | 4 | ||||
| Table IV | 24.3 | 4.9 | 11–35 | 62 |
N.A., not available.
K-Fiber Maturation
One striking feature that emerges from our analysis is the relatively slow initial rate of kMt acquisition (∼1.9 kMt/ min). Others have anticipated a much faster rate (i.e., Merdes and De Mey, 1990; Zhai et al., 1996), although our results are consistent with Rieder and Alexander's (1990) study of initial chromosome attachment in newt lung cells. An association rate of 1.9 kMt/min is too slow to balance the kMt dissociation rate estimated by Zhai et al., 1995, and still maintain 24 Mts/kinetochore at equilibrium, where equilibrium is estimated as the average kMt number at late metaphase (see Table IV). For this reason, the full time course of K-fiber formation cannot be described by a simple model of association/dissociation of kMts from independent binding sites on the kinetochore (see Fig. 6 b). Rather, the results suggest that the rate of kMt acquisition increases during K-fiber formation.
We cannot draw this conclusion directly from the prometaphase time course data without considering kMt numbers at metaphase (Table IV), which effectively provides 62 additional points to the upper right of the plot in Fig. 6 a. This makes it clear that the curve in Fig. 6 b reaches a plateau at far too few kMts. Analysis of the time course is hindered by variability in the data, some of which results from a twofold variation in the potential Mt-binding capacity of the kinetochores on different PtK1 chromosomes (McEwen, B.F., A.B. Heagle, and C.L. Rieder, manuscript in preparation). Another source of scatter is the variation in the density of free Mt plus ends caused by obstructions between the kinetochore and its spindle pole (see Results). It is also likely that the stochastic nature of Mt growth and the association/dissociation reaction contributes to the observed variability.
Our analysis assumes that kMt number has reached equilibrium by late metaphase. Although we have no direct evidence for this, if equilibrium has not been reached, then kMt numbers would still be increasing during late metaphase. In this case, the discrepency between the data and the simple association/dissociation model would be even greater. An additional consideration is the accuracy of our estimation of the kMt saturation level, which is based on measurements of the kinetochore outer plate size and a maximum density of kMt packing (see Rieder, 1982). The kMt acquisition rate required to balance the dissociation rate does decrease if higher values for the saturation level are used in the calculations, but the time course plot is not very sensitive to this parameter. Even with unrealistically large values for kMt saturation (i.e., on the order of 300 kMts), a curve based on the simple association/ dissociation model still fails to fit both the initial velocity and the metaphase distribution (calculations not shown).
Two likely mechanisms by which the association rate could increase during K-fiber formation include an increase in the concentration of free Mt plus ends in the vicinity of the kinetochore and/or cooperativity. In early prometaphase, when many kinetochores first attach to the spindle, the total amount of Mt polymer is about half its value at metaphase (Zhai et al., 1996). Thus the concentration of free Mt plus ends rises during K-fiber formation, with the result that the kMt association rate is also predicted to rise (see Eq. 4 in Materials and Methods). For congression during late prometaphase, the total Mt polymer level is similar to metaphase, but the newly attached kinetochore is generally positioned far from the spindle pole to which it is connected, and hence in a region containing few Mt plus ends emanating from that pole. This number progressively increases as the chromosome congresses to the spindle equator (see McIntosh and Landis, 1971; Brinkley and Cartwright, 1971). In this regard, it is interesting that congressing and monoorienting chromosomes have roughly the same slow initial rate of kMt acquisition.
Cooperativity during K-fiber formation could result from the favorable interactions between the kMts of an individual kinetochore, which make it easier for successive Mt plus ends to bind. Such interactions and mechanical linkages are indicated by the natural bundling of kMts within a K-fiber (e.g., Rieder, 1981a ; McDonald et al., 1992) and by the cohesiveness of this bundle to micromanipulation (e.g., Nicklas et al., 1982).
Recently, Zhai et al. (1996) determined that Mt polymer levels remain constant through prophase, decrease dramatically just after nuclear envelope breakdown, then slowly increase almost back to prophase values by metaphase. The decrease in total polymer immediately after nuclear envelope breakdown is caused by the increased dynamic instability of Mts, which Zhai et al. (1996) observed at the same time. Since the increased dynamic instability persists into anaphase, Zhai et al. (1996) proposed that the recovery of Mt polymer levels is caused by tension-driven polymerization/stabilization of kMts. However, their model assumes that the newly attaching kinetochore of a previously monooriented chromosome saturates rapidly so that during congression, it generally has a full complement of kMts. This assumption conflicts with the results presented here (Fig. 6 and Table I), which favor a model where most kMt polymer is generated by kinetochores slowly capturing and stabilizing longer kMts that reach the metaphase plate. In addition, Zhai et al. (1996) observed that most of the Mt polymer at metaphase remains sensitive to brief nocodazole treatment. These results indicate that the majority of the Mts formed after nuclear envelope breakdown are not kMts. It is possible that the non-kMts are stabilized by the high Mt density within the bipolar spindle.
The Direction a Bioriented Chromosome Moves Is Not Determined by the Relative Numbers of Mts on Its Sister Kinetochores
As a consequence of the slow initial rate of kMt acquisition, the disparity in the Mt number between sister kinetochores of congressing chromosomes is larger than was previously anticipated, and much larger than predicted by the formulation of Hays and Salmon (1990; see Table I). Indeed, in one example, we found 1 kMt “out-pulling” 26, and in another, 2 out-pulling 11. In contrast, the corresponding kinetochore-to-pole distances differ only by a factor of 2–3 (Table I). Thus, poleward force production during congression in somatic cells is not correlated with the product of kMt number and distance to the spindle pole, and in fact appears to be independent of kMt number. This conclusion can be extended to bioriented chromosomes at the metaphase plate, where we observed a slight inverse correlation between kMt number and direction of motion, and no correlation between kMt number and the probability of movement (Table III). Clearly, the number of Mts on attached kinetochores is not important for determining the direction of kinetochore motion in PtK1 cells.
The inconsistency between our data and the predictions of Hays and Salmon (1990) could reflect differences in behavior between meiotic vs. mitotic systems and/or insect vs. vertebrate cells. However, as in grasshopper spermatocytes, destruction of one kinetochore on congressed PtK1 chromosomes also induces the chromosome to move off the spindle equator toward the pole attached to the nonirradiated kinetochore (McNeil and Berns, 1981; Rieder et al., 1995). In addition, premature separation of sister chromatids by laser surgery invariably results in poleward migration of the individual kinetochores (Skibbens et al., 1995; Khodjakov and Rieder, 1996). Thus, in mitotic systems, equatorial positioning of bioriented chromosomes also requires the antagonistic poleward pull of sister kinetochores. Hays and Salmon's data (1990) demonstrate that when kinetochore function is partially destroyed, the distance that chromosomes move from the spindle equator is correlated with the number of attached kMts. However, this correlation between the final chromosome position and kMt number does not establish a cause and effect relationship. An alternative explanation is that both the number of Mts and the number of force-producing sites on the kinetochore are reduced by irradiation, but only the latter determines the final chromosome location (see Rieder and Alexander, 1990). This view is consistent with both our results and those of Hays and Salmon (1990).
Our results are inconsistent with traction fiber models which postulate that the molecules producing poleward force for chromosome motion reside along the K-fiber (Hays et al., 1982; Pickett-Heaps, 1986; Fuge, 1989; Pickett-Heaps et al., 1996). These models have been largely abandoned in favor of the poleward force being generated primarily at the kinetochore by either kMt depolymerization or minus-end–directed Mt motor molecules, and secondarily at the spindle pole by poleward flux (reviewed by McIntosh and Pfarr, 1991; Rieder and Salmon, 1994; Desai and Mitchison, 1995; Inoue and Salmon, 1995; Vernos and Karsenti, 1996). However, like the traction fiber model, all of these mechanisms also predict that force production will depend on kMt number. For kMt depolymerization, the dependence should be linear, since each kMt releases the same amount of chemical energy upon depolymerization. For Mt motor–driven motion, the relationship would be more complex because the same amount of force could be generated by several motor molecules interacting with one kMt as by each motor molecule interacting with a different kMt (see Rieder and Alexander, 1990). Nevertheless, when the kMt number is well below saturation, as it is for the P kinetochore on congressing chromosomes, it is probable that many of the motor molecules are unable to form productive interactions with a kMt, either because distances between motor molecules and the nearest kMt are too large, and/or because of too much competition for binding sites along each kMt. Thus, for motor molecules, the kMt dependence of poleward force production should be determined by the limiting factor, either the availability of motors or the number of kMts.
Chromosome motion is also effected by polar ejection forces, which are generated external to the kinetochore and push the chromosome away from the nearest spindle pole (reviewed in Rieder and Salmon, 1994; Khodjakov and Rieder, 1996; Vernos and Karsenti, 1996). During congression, polar ejection forces aid the leading kinetochore in moving the chromosome toward the metaphase plate. However, this does not explain the lack of correlation between kMt number and direction of motion because, in the moment before biorientation, the monooriented chromosome is positioned at or oscillates about a point where the P force generated by the kinetochore balances the polar ejection forces. If poleward force production were linearly dependent on the kMt number, then 1 kMt binding to the sister kinetochore would not drastically alter the position of the balance point, and chromosomes would generally move to the spindle equator slowly through a series of new balance points as the leading kinetochore gradually acquires more kMts. Instead, chromosomes congress steadily to the spindle equator with few interruptions (e.g., Skibbens et al., 1993; Khodjakov and Rieder, 1996), indicating that the balance between astral ejection forces and the P force of a monooriented chromosome is drastically changed by the binding of a single kMt to the unattached kinetochore, even when the originally attached kinetochore has nearly a full complement of kMts.
On the basis of in vitro results, Hyman and Mitchison (1991a,b) postulated that kMt acquisition partially inhibits poleward force generation by deactivating a kinetochore minus-end–directed Mt motor such as cytoplasmic dynein. This model is consistent with the data in Tables I and III, because it predicts an inverse relationship between the kMt number and poleward force generation. However, the model also predicts that the distance between monooriented chromosomes and their attached spindle poles should be directly related to kMt number because kinetochores with fewer kMts would generate more P force, and hence on average be able to approach closer to the spindle pole in their oscillation cycles before the tension level from astral ejection forces causes switching into neutral (see Salmon, 1989). We failed to detect a correlation between kMt number and distance from the spindle pole (data not shown), in agreement with Cassimeris et al. (1994), who failed to detect such a correlation in monopolar newt lung spindles. Furthermore, the dynein-deactivation model fails to account for how a single kinetochore can initiate congression after being prematurely separated from its sister by laser surgery (Khodjakov et al., 1997) or how kinetochore fragments from an unreplicated genome migrate to the spindle equator (Wise and Brinkley, 1997).
An Alternative Model for Congression
When laser surgery is used to separate chromatids of congressing chromosomes, the trailing kinetochore abruptly stops moving AP and remains stationary for a variable length of time (up to 60 s) before switching to P motion (Khodjakov and Rieder, 1996). Thus, PtK1 kinetochores that are moving AP are in a “neutral” (i.e., non–force-producing) state (see also Waters et al., 1996b ), and there is an obligatory pause before the kinetochore is able to switch back to the P force–producing state. Thus, congression is not a tug of war between two poleward producing forces acting on the sister kinetochores: i.e., the leading kinetochore does not outpull the trailing kinetochore (see also Skibbens et al., 1993). Rather, the initial attachment of the unattached kinetochore on a monooriented chromosome produces a sudden change in tension level that induces the previously attached kinetochore to switch into the neutral state (see Skibbens et al., 1993, Rieder and Salmon, 1994, and Skibbens et al., 1995). Aided by the astral ejection forces, the attaching (now leading) kinetochore would then be able to move the chromosome a considerable distance toward the metaphase plate before the trailing kinetochore switches back into the force-producing or P motion state. After a brief reversal of direction (most PtK1 and newt lung chromosomes show at least one oscillation during congression, as seen in Fig. 2 b) that is opposed by the polar ejection forces, the kinetochore closest to its pole again switches into the neutral state. By the time it is able to switch back to the force-producing state for a second time, the chromosome is near the metaphase plate and the leading kinetochore undoubtedly has acquired several kMts, since the rate of kMt acquisition increases with time. Thus, a mechanism based on a tension-sensitive, time-delayed switch, participation of polar ejection forces, and deviation from a strict linear dependence of force production upon kMt number explains how congression can occur, even when there is a wide disparity of kMt numbers between sister kinetochores. In fact, the kMt number appears to have very little to do with either congression or the tendency for a kinetochore to be in the P state.
Distribution of kMt Number after Anaphase Onset and Taxol Treatment
The increase in kMt number we observed during early anaphase appears to arise from a four to fivefold increase in the stability of kMts that occurs with anaphase onset (Zhai et al., 1995). At first glance, our results seem at variance with others who have observed that kMt number decreases during anaphase (e.g., Jensen, 1982). Such a decrease is expected because the number of free Mts in the vicinity of the kinetochore decreases for mammalian cells during the course of anaphase (McIntosh and Landis, 1971; Brinkley and Cartwright, 1971), which in turn will reduce the rate of kMt acquisition. During early anaphase, however, the chromosomes still display brief periods of AP motion (Bajer, 1982; Skibbens et al., 1993), and they are able to incorporate new Mts into their K-fibers (Wadsworth et al., 1989). These data indicate that in contrast to late anaphase, the concentration of dynamic Mt ends is still sufficient to produce significant astral ejection forces and kMt association. Therefore, it is probable that the rate of kMt association, which is dependent on the concentration of free Mt plus ends, is also initially maintained at the metaphase level. Thus, the kMt number should show a transient rise with anaphase onset because of the decreased dissociation rate, followed by a steady decline as the number of free Mt plus ends decreases. Indeed, this seems to be indicated when our results for early anaphase are compared with those of McDonald et al. (1992), who found metaphase levels of kMts in PtK1 cells during mid-anaphase and less than metaphase levels at late anaphase. The transitory nature of the kMt increase is also indicated by the greater variation (i.e., higher standard deviation) in kMt number during early anaphase, as compared with late metaphase (Table IV).
When metaphase PtK1 cells are treated for 10 min with a concentration of taxol that is sufficient to block anaphase onset, the kMt number increases to the level seen in early anaphase (Table IV). From this, we conclude that a full complement of kMts is not sufficient to induce anaphase onset, and that the checkpoint mechanism does not monitor kMt number. Alternative possibilities for effectors of the metaphase/anaphase transition include dynamic instability of kMts and tension produced across the kinetochore (Rieder et al., 1994; Nicklas et al., 1995; Waters et al., 1996a ).
The increased kMt number observed upon taxol treatment could be the result of a decrease in the kMt dissociation constant. Alternatively, the increase could also be the result of more free Mt plus ends in the vicinity of the kinetochores, since taxol promotes Mt polymerization as well as Mt stability (reviewed in Wilson and Jordan, 1994). The increase in the kMt number is transient, however, as indicated by the lower kMt numbers for the 60-min treatment (see Results) and by the larger standard deviation of kMt numbers from taxol-treated cells compared with untreated metaphase cells (Table IV). The decrease after longer incubations could indicate a subsequent drop in the concentration of free Mt plus ends near the kinetochores since Mts no longer turn over frequently. The reduction in kMt number could also result from the eventual depolymerization of kMts from the minus end, since this activity is known to continue in the presence of taxol (Waters et al., 1996a ).
Acknowledgments
We thank Richard Cole for technical advice concerning video microscopy and chromosome tracking, and Drs. Alexey Khodjakov and Jeff Ault for critical comments on the manuscript and stimulating conversations during the project. We also thank Andrea Pouchak for technical assistance and Dr. Pawel Penczek and Christian Whiting for aid in interpreting the time course data. Eq. 3 was solved by Christian Whiting, producing Eqs. 5 and 9, and the empirical fit described by Eq. 8 was suggested by Dr. Penczek.
This study was supported by National Science Foundation grant No. MCB 94 20772 (to B.F. McEwen), and by National Institutes of Health grants GMS 40198 (to C.L. Rieder) and NCRR/BTP P41-01219, which partly supports the Wadsworth Center's Biological Microscopy and Image Reconstruction (BMIR) Facility as a National Biotechnological Resource. The video light microscopic component of BMIR is also supported by the Wadsworth Center as a core facility. The study also made use of the Visualization and Modeling for Biological Complexity facility funded by National Science Foundation grant No. BIR 9219043 (to J. Frank).
Footnotes
1. Abbreviations used in this paper: AP, away from the pole; 3D, three- dimensional; K-fiber, kinetochore fiber; kMts, kinetochore microtubules; Mts, microtubules; P, poleward.
Address all correspondence to Dr. Bruce F. McEwen, Division of Molecular Medicine, Wadsworth Center, New York State Department of Health, P.O. Box 509, Albany, NY 12201-0509. Tel.: (518) 474-9120. Fax: (518) 474-7992. E-mail: bruce@wadsworth.org
References
- Bajer AS. Functional autonomy of monopolar spindle and evidence for oscillatory movement in mitosis. J Cell Biol. 1982;93:33–48. doi: 10.1083/jcb.93.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley BR, Cartwright J., Jr Ultrastructural analysis of mitotic spindle elongation in mammalian cells in vitro: direct microtubule counts. J Cell Biol. 1971;50:416–431. doi: 10.1083/jcb.50.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris L, Rieder CL, Rupp G, Salmon ED. Stability of microtubule attachment to metaphase kinetochores in PtK1cells. J Cell Sci. 1990;96:9–15. doi: 10.1242/jcs.96.1.9. [DOI] [PubMed] [Google Scholar]
- Cassimeris L, Rieder CL, Salmon ED. Microtubule assembly and kinetochore directional instability in vertebrate monopolar spindles: implications for the mechanism of chromosome congression. J Cell Sci. 1994;107:285–297. doi: 10.1242/jcs.107.1.285. [DOI] [PubMed] [Google Scholar]
- Desai A, Mitchison TJ. A new role for motor proteins as couplers to depolymerizing microtubules. J Cell Biol. 1995;128:1–4. doi: 10.1083/jcb.128.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuge H. Traction fibers in chromosome movement: the pros and cons. Biol Cell. 1989;66:209–213. [PubMed] [Google Scholar]
- Gorbsky GJ, Sammak PJ, Borisy GG. Microtubule dynamics and chromosome motion visualized in living anaphase cells. J Cell Biol. 1988;106:1185–1192. doi: 10.1083/jcb.106.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science (Wash DC) 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Hays TS, Salmon ED. Poleward force at the kinetochore in metaphase depends on the number of kinetochore microtubules. J Cell Biol. 1990;110:391–404. doi: 10.1083/jcb.110.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays TS, Wise D, Salmon ED. Traction force on a kinetochore at metaphase acts as a linear function of kinetochore fiber length. J Cell Biol. 1982;93:374–382. doi: 10.1083/jcb.93.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindus LA. 2K images from a 1K camera: high resolution at lower cost. Advanced Imaging. 1992;7:52–53. [Google Scholar]
- Hyman AA, Mitchison TJ. Two different microtubule-based motor activities with opposite polarities in kinetochores. Nature (Lond) 1991a;351:206–211. doi: 10.1038/351206a0. [DOI] [PubMed] [Google Scholar]
- Hyman AA, Mitchison TJ. Regulation of the direction of chromosome movement. Cold Spring Harbor Symp Quant Biol. 1991b;56:745–750. doi: 10.1101/sqb.1991.056.01.083. [DOI] [PubMed] [Google Scholar]
- Hyman AA, Mitchison TJ. Molecular basis of chromosome movement. Curr Opin Struct Biol. 1992;2:275–279. [Google Scholar]
- Inoue S, Salmon ED. Force generation by microtubule assembly/ disassembly in mitosis and related movements. Mol Biol Cell. 1995;6:1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CG. Dynamics of spindle microtubule organization: kinetochore fiber microtubules of plant endosperm. J Cell Biol. 1982;92:540–558. doi: 10.1083/jcb.92.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MA, Thrower D, Wilson L. Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles: implications for the role of microtubule dynamics in mitosis. J Cell Sci. 1992;102:401–416. doi: 10.1242/jcs.102.3.401. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL. Kinetochores moving away from their associated pole do not exert a significant pushing force on the chromosome. J Cell Biol. 1996;135:315–327. doi: 10.1083/jcb.135.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Cole RW, McEwen BF, Buttle KF, Rieder CL. Chromosome fragments possessing only one kinetochore can congress to the spindle equator. J Cell Biol. 1997;136:229–240. doi: 10.1083/jcb.136.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko M, Leith A. Sterecon—three-dimensional reconstructions from stereoscopic contouring. J Struct Biol. 1996;116:93–98. doi: 10.1006/jsbi.1996.0016. [DOI] [PubMed] [Google Scholar]
- McDonald KL, O'Toole ET, Mastronarde DN, McIntosh JR. Kinetochore microtubules in PTK cells. J Cell Biol. 1992;118:369–383. doi: 10.1083/jcb.118.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, B.F., G. Osorio, R. Cole, and C.L. Rieder. 1995. Same-cell correlative video light microscopy/electron microscopy tomography: an approach to understanding kinetochore behavior during mitosis. In Proceedings in Microscopy and Microanalysis. G.W. Bailey, M.H. Ellisman, R.A. Hennigar, and N.J. Zaluzec, editors. Jones and Begell Publishing, New York. 744–745.
- McIntosh JR, Landis SC. The distribution of spindle microtubules during mitosis in cultured human cells. J Cell Biol. 1971;49:468–497. doi: 10.1083/jcb.49.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JR, Cande Z, Snyder J, Vanderslice K. Studies on the mechanism of mitosis. Ann NY Acad Sci. 1975;253:407–427. doi: 10.1111/j.1749-6632.1975.tb19217.x. [DOI] [PubMed] [Google Scholar]
- McIntosh JR, Pfarr CM. Mitotic motors. J Cell Biol. 1991;115:577–585. doi: 10.1083/jcb.115.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PA, Berns MW. Chromosome behavior after laser microirradiation of a single kinetochore in mitotic PtK2cells. J Cell Biol. 1981;88:543–553. doi: 10.1083/jcb.88.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A, De Mey J. The mechanism of kinetochore-spindle attachment and polewards movement analyzed in PtK2cells at the prophase-prometaphase transition. Eur J Cell Biol. 1990;53:313–325. [PubMed] [Google Scholar]
- Mitchison TJ, Salmon ED. Poleward kinetochore fiber movement occurs during both metaphase and anaphase-A in newt lung cell mitosis. J Cell Biol. 1992;119:569–582. doi: 10.1083/jcb.119.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas RB. The motor for poleward chromosome movement in anaphase is in or near the kinetochore. J Cell Biol. 1989;109:2245–2255. doi: 10.1083/jcb.109.5.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas RB, Ward SC. Elements of error correction in mitosis: microtubule capture, release, and tension. J Cell Biol. 1994;126:1241–1253. doi: 10.1083/jcb.126.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas RB, Kubai DF, Hays TS. Spindle microtubules and their mechanical associations after micromanipulation in anaphase. J Cell Biol. 1982;95:91–104. doi: 10.1083/jcb.95.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett-Heaps JD. Mitotic mechanisms: an alternative view. Trends Biochem Sci. 1986;11:504–508. [Google Scholar]
- Pickett-Heaps JD, Forer A, Spurck T. Rethinking anaphase: where “Pac-Man” fails and why a role for the spindle matrix is likely. Protoplasma. 1996;192:1–10. [Google Scholar]
- Rieder CL. The structure of the cold-stable kinetochore fiber in metaphase PtK1cells. Chromosoma (Berl) 1981a;84:145–158. doi: 10.1007/BF00293368. [DOI] [PubMed] [Google Scholar]
- Rieder CL. Thick and thin serial sectioning for the three-dimensional reconstruction of biological ultrastructure. Methods Cell Biol. 1981b;22:215–249. doi: 10.1016/s0091-679x(08)61879-8. [DOI] [PubMed] [Google Scholar]
- Rieder CL. The formation, structure and composition of the mammalian kinetochore and kinetochore fiber. Int Rev Cytol. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Alexander SP. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol. 1990;110:81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, C.L., and R.W. Cole. 1997 Perfusion chambers for high-resolution video light microscopic studies of vertebrate cell monolayers: some considerations and a design. In Methods in Cell Biology: Video Microscopy. G. Sluder and D.E. Wolf, editors. Academic Press, New York. In press. [DOI] [PubMed]
- Rieder CL, Salmon ED. Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle. J Cell Biol. 1994;124:223–233. doi: 10.1083/jcb.124.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Schultz A, Cole R, Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Cole RW, Khodjakov A, Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos U-P. Light and electron microscopy of rat kangaroo cells in mitosis. II. Kinetochore structure and function. Chromosoma (Berl) 1973;41:195–220. doi: 10.1007/BF00319696. [DOI] [PubMed] [Google Scholar]
- Salmon, E.D. 1989. Metaphase chromosome congression and anaphase poleward movement. In Kinesin, Dynein, and Microtubule Dynamics. F.D. Warner and J.R. McIntosh, editors. Alan R. Liss Inc., New York. 431–440.
- Skibbens RV, Skeen VP, Salmon ED. Directional instability of kinetochore motility during chromosome congression and segregation in mitotic newt lung cells: a push-pull mechanism. J Cell Biol. 1993;122:859–875. doi: 10.1083/jcb.122.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibbens RV, Rieder CL, Salmon ED. The behavior of prometaphase kinetochores after separation from the centromere and chromosome arms by laser microsurgery: evidence that kinetochore directional instability and position is regulated by tension. J Cell Sci. 1995;108:2537–2548. doi: 10.1242/jcs.108.7.2537. [DOI] [PubMed] [Google Scholar]
- Vernos I, Karsenti E. Motors involved in spindle assembly and chromosome segregation. Curr Opin Cell Biol. 1996;8:4–9. doi: 10.1016/s0955-0674(96)80041-x. [DOI] [PubMed] [Google Scholar]
- Wadsworth P, Shelden E, Rupp G, Rieder CL. Biotin-tubulin incorporates into kinetochore fiber microtubules during early but not late anaphase. J Cell Biol. 1989;109:2257–2265. doi: 10.1083/jcb.109.5.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JC, Cole RW, Rieder CL. The force-producing mechanism for centrosome separation during spindle formation in vertebrates is intrinsic to each aster. J Cell Biol. 1993;122:361–372. doi: 10.1083/jcb.122.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JC, Mitchison TJ, Rieder CL, Salmon ED. The kinetochore microtubule minus-end disassembly associated with poleward flux produces a force that can do work. Mol Biol Cell. 1996a;7:1547–1558. doi: 10.1091/mbc.7.10.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JC, Skibbens RV, Salmon ED. Oscillating mitotic newt lung cell kinetochores are, on average, under tension and rarely push. J Cell Sci. 1996b;109:2823–2831. doi: 10.1242/jcs.109.12.2823. [DOI] [PubMed] [Google Scholar]
- Wendell KL, Wilson L, Jordan MA. Mitotic block in HeLa cells by vinblastine: ultrastructural changes in kinetochore-microtubule attachment and in centrosomes. J Cell Sci. 1993;104:261–274. doi: 10.1242/jcs.104.2.261. [DOI] [PubMed] [Google Scholar]
- Wilson, L., and M.A. Jordan. 1994 Pharmacological probes of microtubule function. In Microtubules. J.S. Hyams, and C.W. Lloyd, editors. Wiley-Liss, New York. pp. 59–83.
- Wise DA, Brinkley BR. Mitosis in cells with unreplicated genomes (MUGs): spindle assembly and behavior of centromere fragments. Cell Motil Cytoskeleton. 1997;36:291–302. doi: 10.1002/(SICI)1097-0169(1997)36:3<291::AID-CM9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Kronebusch PJ, Borisy GG. Kinetochore microtubule dynamics and the metaphase-anaphase transition. J Cell Biol. 1995;131:721–734. doi: 10.1083/jcb.131.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y, Kronebusch PJ, Simon PM, Borisy GG. Microtubule dynamics at the G2/M transition: abrupt breakdown of cytoplasmic microtubules at nuclear envelope breakdown and implications for spindle morphogenesis. J Cell Biol. 1996;135:201–214. doi: 10.1083/jcb.135.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]