Abstract
The phospholipase C (PLC) gene of Pseudomonas aeruginosa encodes a heat-labile secreted hemolysin which is part of a Pi-regulated operon. The structural gene for PLC, plcS, was mutated in vitro by insertion of a tetracycline resistance gene cartridge. Gene replacement techniques were used to introduce the mutated plcS gene into the P. aeruginosa chromosome in place of the wild-type gene. The precise replacement of wild-type sequences by mutant sequences was confirmed by Southern hybridization. The mutant strain, designated PLC S, is nonhemolytic and lacks a 78-kilodalton protein corresponding to the size of the wild-type PLC. However, there is an additional phospholipase activity present in PLC S capable of hydrolyzing p-nitrophenylphosphorylcholine, a synthetic PLC substrate, and phosphatidylcholine. This enzymatic activity is not a result of a truncated product produced from the mutated plcS gene. The phospholipase activity of PLC S was identified as a nonhemolytic PLC.
Full text
PDF

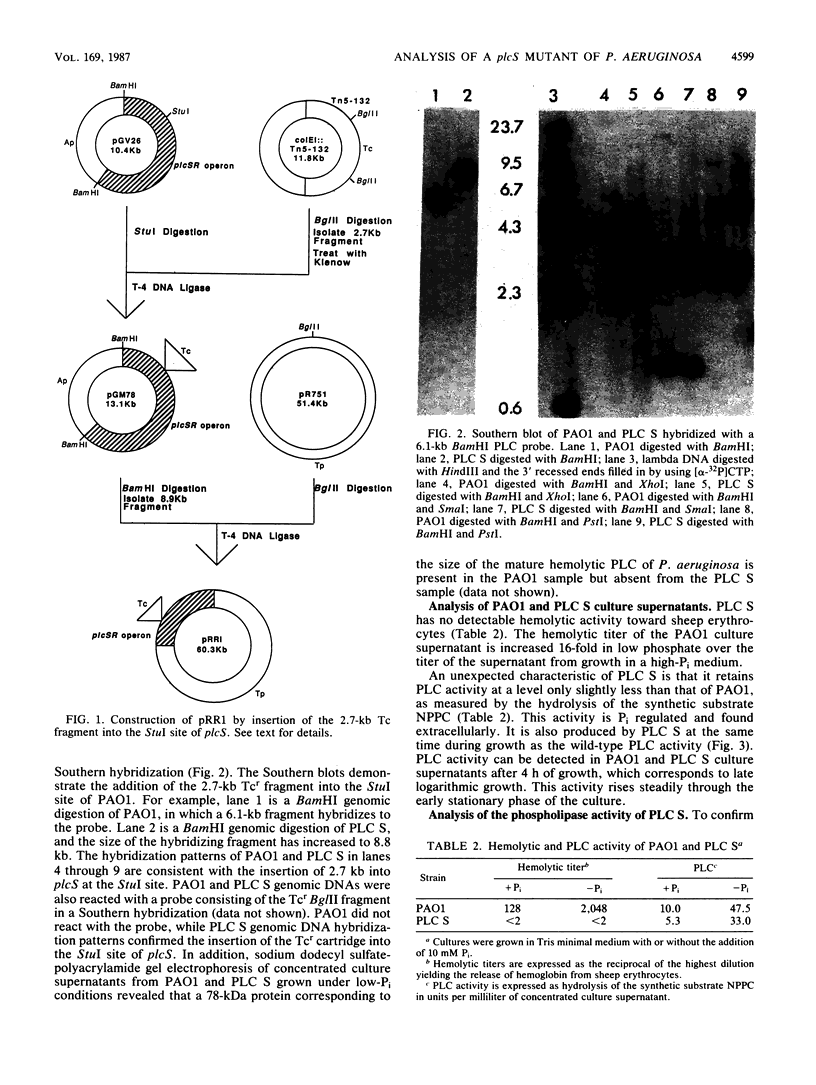
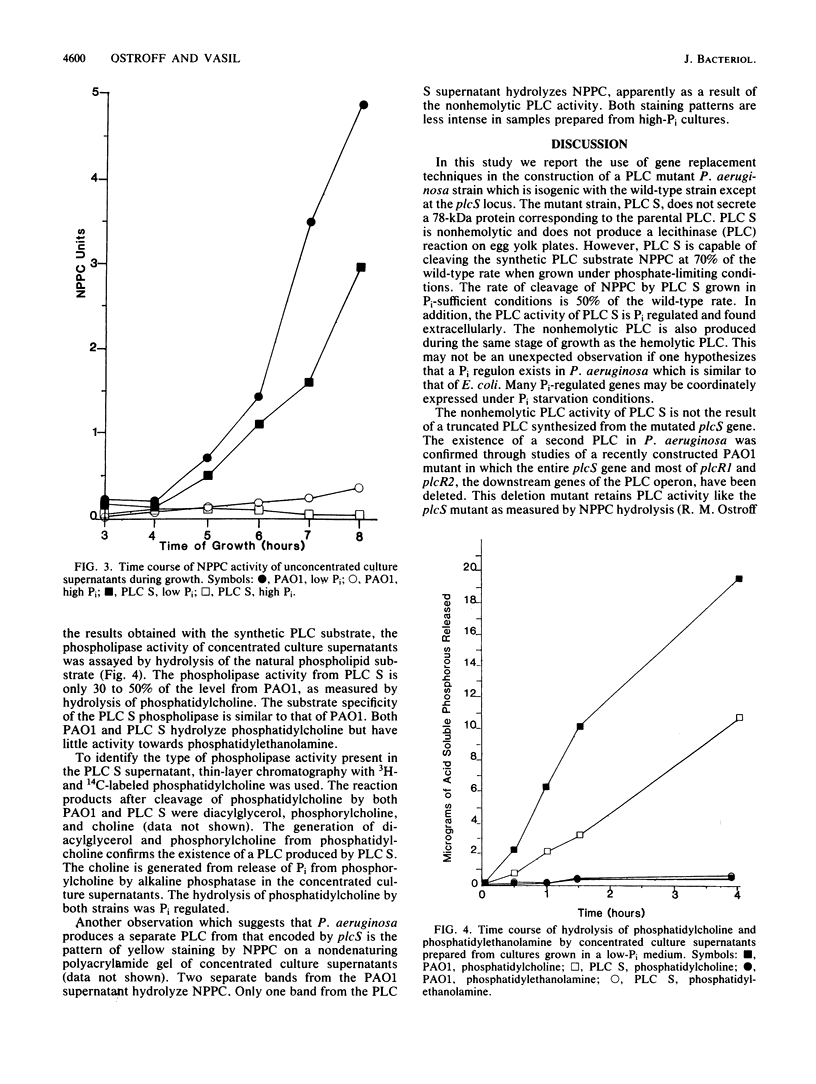

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Egner C., Hirschel B. J., Howard J., Johnsrud L., Jorgensen R. A., Tlsty T. D. Insertion, excision, and inversion of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):115–123. doi: 10.1101/sqb.1981.045.01.020. [DOI] [PubMed] [Google Scholar]
- Berka R. M., Vasil M. L. Phospholipase C (heat-labile hemolysin) of Pseudomonas aeruginosa: purification and preliminary characterization. J Bacteriol. 1982 Oct;152(1):239–245. doi: 10.1128/jb.152.1.239-245.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- ESSELMANN M. T., LIU P. V. Lecithinase production by gramnegative bacteria. J Bacteriol. 1961 Jun;81:939–945. doi: 10.1128/jb.81.6.939-945.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W., Schollmeier K. Nucleotide sequence of the Tn10 encoded tetracycline resistance gene. Nucleic Acids Res. 1983 Jan 25;11(2):525–539. doi: 10.1093/nar/11.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Morgan A. F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979 Mar;43(1):73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Watson J. M., Haas D., Leisinger T. Genetic and molecular characterization of the Pseudomonas plasmid pVS1. Plasmid. 1984 May;11(3):206–220. doi: 10.1016/0147-619x(84)90027-1. [DOI] [PubMed] [Google Scholar]
- Kurioka S., Liu P. V. Improved assay method for phospholipase C. Appl Microbiol. 1967 May;15(3):551–555. doi: 10.1128/am.15.3.551-555.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurioka S., Matsuda M. Phospholipase C assay using p-nitrophenylphosphoryl-choline together with sorbitol and its application to studying the metal and detergent requirement of the enzyme. Anal Biochem. 1976 Sep;75(1):281–289. doi: 10.1016/0003-2697(76)90078-6. [DOI] [PubMed] [Google Scholar]
- Lory S., Tai P. C. Characterization of the phospholipase C gene of Pseudomonas aeruginosa cloned in Escherichia coli. Gene. 1983 Apr;22(1):95–101. doi: 10.1016/0378-1119(83)90068-9. [DOI] [PubMed] [Google Scholar]
- Meyer R. J., Shapiro J. A. Genetic organization of the broad-host-range IncP-1 plasmid R751. J Bacteriol. 1980 Sep;143(3):1362–1373. doi: 10.1128/jb.143.3.1362-1373.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard A. E., Vasil M. L. Nucleotide sequence and expression of a phosphate-regulated gene encoding a secreted hemolysin of Pseudomonas aeruginosa. J Bacteriol. 1986 Jul;167(1):291–298. doi: 10.1128/jb.167.1.291-298.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B. F., Tai P. C., Pritchard A. E., Vasil M. L. Nucleotide sequences and expression in Escherichia coli of the in-phase overlapping Pseudomonas aeruginosa plcR genes. J Bacteriol. 1987 Oct;169(10):4602–4607. doi: 10.1128/jb.169.10.4602-4607.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Hayden C. Secretion of phospholipase C by Pseudomonas aeruginosa. Infect Immun. 1979 Aug;25(2):558–564. doi: 10.1128/iai.25.2.558-564.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Berka R. M., Gray G. L., Nakai H. Cloning of a phosphate-regulated hemolysin gene (phospholipase C) from Pseudomonas aeruginosa. J Bacteriol. 1982 Oct;152(1):431–440. doi: 10.1128/jb.152.1.431-440.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Chamberlain C., Grant C. C. Molecular studies of Pseudomonas exotoxin A gene. Infect Immun. 1986 May;52(2):538–548. doi: 10.1128/iai.52.2.538-548.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L. Pseudomonas aeruginosa: biology, mechanisms of virulence, epidemiology. J Pediatr. 1986 May;108(5 Pt 2):800–805. doi: 10.1016/s0022-3476(86)80748-x. [DOI] [PubMed] [Google Scholar]