Abstract
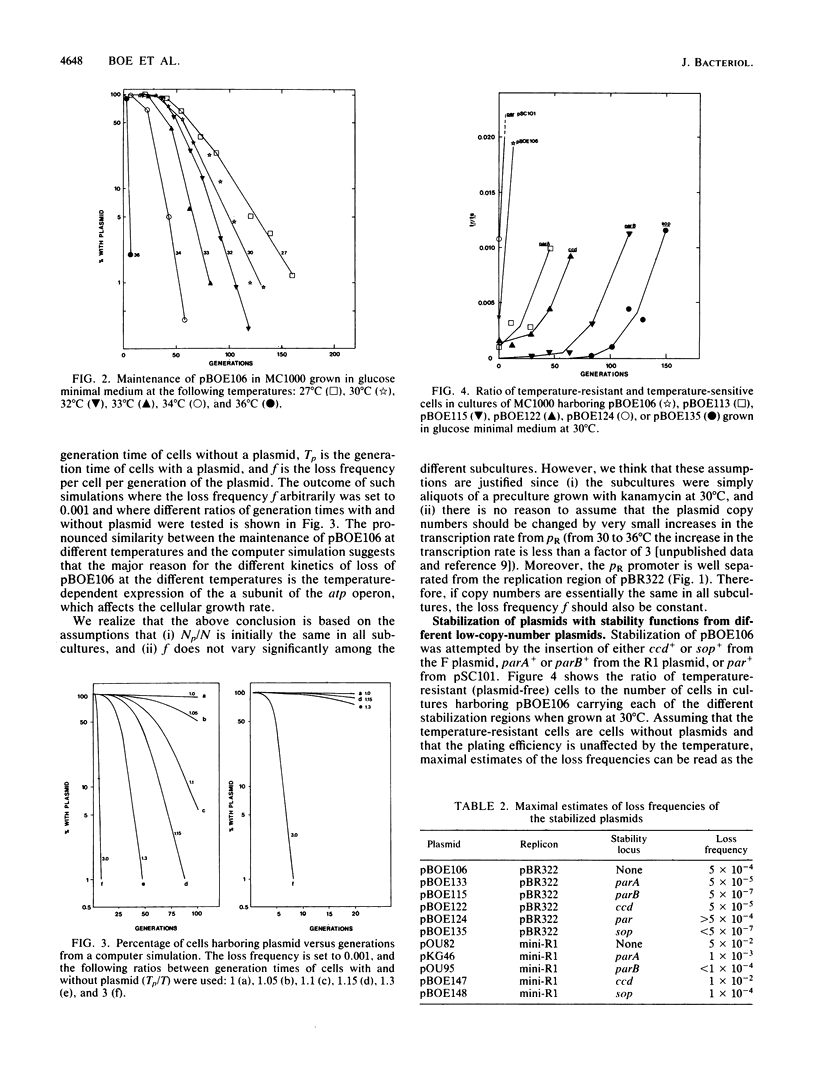
Plasmid stabilization mediated by the parA+ and parB+ genes of the R1 plasmid and the ccd+ and sop+ genes of the F plasmid was tested on a mini-R1 plasmid and a pBR322 plasmid derivative. The mini-R1 plasmid is thought to be unstably inherited owing to a low copy number and to random segregation of the plasmid at cell division, whereas cells harboring the pBR322 derivative used in this work are lost through competition with plasmid-free cells, mainly as a result of the shorter generation time of cells without plasmids. The pBR322 derivative carries a fusion between part of the atp operon of Escherichia coli and the bacteriophage lambda pR promoter, and the cI857 repressor gene. The insertion of sop+ from the F plasmid or parB+ from the R1 plasmid reduced the loss frequency by a factor of 10(3) for the pBR322 derivative and by at least a factor of 10(2) for the mini-R1 plasmid. Insertion of parA+ from the R1 plasmid decreased the loss frequency of the pBR322 derivative by a factor of 10 and that of the mini-R1 plasmid by a factor of 50. When ccd+ from the F plasmid was inserted, the loss frequency of the pBR322 derivative was decreased by a factor of 10, but it had only a marginal effect on the stability of the mini-R1 plasmid. In no case was any significant structural instability of the plasmids observed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Gerdes K., Molin S. Partitioning of plasmid R1. Structural and functional analysis of the parA locus. J Mol Biol. 1986 Aug 5;190(3):269–279. doi: 10.1016/0022-2836(86)90001-x. [DOI] [PubMed] [Google Scholar]
- Gerdes K., Rasmussen P. B., Molin S. Unique type of plasmid maintenance function: postsegregational killing of plasmid-free cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3116–3120. doi: 10.1073/pnas.83.10.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchliffe E., Kuempel P. L., Masters M. Escherichia coli minichromosomes containing the pSC101 partitioning locus are stably inherited. Plasmid. 1983 May;9(3):286–297. doi: 10.1016/0147-619x(83)90006-9. [DOI] [PubMed] [Google Scholar]
- Jaffé A., Ogura T., Hiraga S. Effects of the ccd function of the F plasmid on bacterial growth. J Bacteriol. 1985 Sep;163(3):841–849. doi: 10.1128/jb.163.3.841-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Larsen J. E., Gerdes K., Light J., Molin S. Low-copy-number plasmid-cloning vectors amplifiable by derepression of an inserted foreign promoter. Gene. 1984 Apr;28(1):45–54. doi: 10.1016/0378-1119(84)90086-6. [DOI] [PubMed] [Google Scholar]
- Meacock P. A., Cohen S. N. Partitioning of bacterial plasmids during cell division: a cis-acting locus that accomplishes stable plasmid inheritance. Cell. 1980 Jun;20(2):529–542. doi: 10.1016/0092-8674(80)90639-x. [DOI] [PubMed] [Google Scholar]
- Nielsen J., Hansen F. G., Hoppe J., Friedl P., von Meyenburg K. The nucleotide sequence of the atp genes coding for the F0 subunits a, b, c and the F1 subunit delta of the membrane bound ATP synthase of Escherichia coli. Mol Gen Genet. 1981;184(1):33–39. doi: 10.1007/BF00271191. [DOI] [PubMed] [Google Scholar]
- Ogura T., Hiraga S. Mini-F plasmid genes that couple host cell division to plasmid proliferation. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4784–4788. doi: 10.1073/pnas.80.15.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T., Hiraga S. Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell. 1983 Feb;32(2):351–360. doi: 10.1016/0092-8674(83)90454-3. [DOI] [PubMed] [Google Scholar]
- Skogman G., Nilsson J., Gustafsson P. The use of a partition locus to increase stability of tryptophan-operon-bearing plasmids in Escherichia coli. Gene. 1983 Aug;23(2):105–115. doi: 10.1016/0378-1119(83)90042-2. [DOI] [PubMed] [Google Scholar]
- Summers D. K., Sherratt D. J. Multimerization of high copy number plasmids causes instability: CoIE1 encodes a determinant essential for plasmid monomerization and stability. Cell. 1984 Apr;36(4):1097–1103. doi: 10.1016/0092-8674(84)90060-6. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., Michelsen O., Sørensen L., McCarthy J. E. Proton conduction by subunit a of the membrane-bound ATP synthase of Escherichia coli revealed after induced overproduction. EMBO J. 1985 Sep;4(9):2357–2363. doi: 10.1002/j.1460-2075.1985.tb03939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., van Deurs B. Physiological and morphological effects of overproduction of membrane-bound ATP synthase in Escherichia coli K-12. EMBO J. 1984 Aug;3(8):1791–1797. doi: 10.1002/j.1460-2075.1984.tb02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


