Abstract
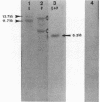
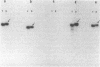
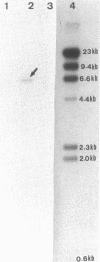
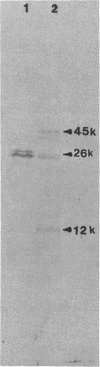
This report examines the genetic basis for Salmonella typhimurium Q1 enterotoxin production. A 918-base-pair XbaI-HincII fragment of plasmid pJM17, composed of cholera toxin (CT) coding sequences (ctxAB), was used as a gene probe. With this probe, the S. typhimurium enterotoxin was identified on a 6.3-kilobase EcoRI-PstI fragment of chromosomal DNA from plasmidless strain Q1. We cloned this 6.3-kilobase fragment into Escherichia coli RR1. The genetic map of the cloned Salmonella enterotoxin (stx) gene was similar but not identical to the CT and E. coli heat-labile enterotoxin genes. By using synthetic oligonucleotides derived from the sequences of CT subunits A (ctxA) and B (ctxB), it was revealed that there were some conserved regions of DNA encoding the enterotoxins of strain Q1 and Vibrio cholerae. Expression of the cloned stx gene in minicells and subsequent Western blot (immunoblot) analysis with CT antitoxin demonstrated that the Salmonella enterotoxin had two or more subunits with molecular sizes of 45, 26, and 12 kilodaltons. Crude cell lysates of E. coli RR1(pCHP4), containing the cloned Salmonella enterotoxin gene, elicited fluid secretion in ligated rabbit intestinal loops and firm induration in rabbit skin. Both of these enterotoxic responses were neutralized by antisera specific for CT. Mucosal tissue from positive intestinal loops contained elevated levels of cyclic AMP. These data suggest some evolutionary relatedness between the enterotoxin genes of S. typhimurium and V. cholerae.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clements J. D., Finkelstein R. A. Immunological cross-reactivity between a heat-labile enterotoxin(s) of Escherichia coli and subunits of Vibrio cholerae enterotoxin. Infect Immun. 1978 Sep;21(3):1036–1039. doi: 10.1128/iai.21.3.1036-1039.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donta S. T., Viner J. P. Inhibition of the steroidogenic effects of cholera and heat-labile Escherichia coli enterotoxins by GM1 ganglioside: evidence for a similar receptor site for the two toxins. Infect Immun. 1975 May;11(5):982–985. doi: 10.1128/iai.11.5.982-985.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Formal S. B., Dammin G. J., Collins H. Pathogenesis of salmonellosis. Studies of fluid secretion, mucosal invasion, and morphologic reaction in the rabbit ileum. J Clin Invest. 1973 Feb;52(2):441–453. doi: 10.1172/JCI107201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Gots R. E., Charney A. N., Greenough W. B., 3rd, Formal S. B. Pathogenesis of Salmonella-mediated intestinal fluid secretion. Activation of adenylate cyclase and inhibition by indomethacin. Gastroenterology. 1975 Dec;69(6):1238–1245. [PubMed] [Google Scholar]
- Gill D. M., Clements J. D., Robertson D. C., Finkelstein R. A. Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infect Immun. 1981 Sep;33(3):677–682. doi: 10.1128/iai.33.3.677-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Richardson S. H. Adenosine diphosphate-ribosylation of adenylate cyclase catalyzed by heat-labile enterotoxin of Escherichia coli: comparison with cholera toxin. J Infect Dis. 1980 Jan;141(1):64–70. doi: 10.1093/infdis/141.1.64. [DOI] [PubMed] [Google Scholar]
- Hejtmancik K. E., Peterson J. W., Markel D. E., Kurosky A. Radioimmunoassay for the antigenic determinants of cholera toxin and its components. Infect Immun. 1977 Sep;17(3):621–628. doi: 10.1128/iai.17.3.621-628.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. E., Payne W. L., Zon G., Moseley S. L. Synthetic oligodeoxyribonucleotide probes for detecting heat-stable enterotoxin-producing Escherichia coli by DNA colony hybridization. Appl Environ Microbiol. 1985 Nov;50(5):1187–1191. doi: 10.1128/aem.50.5.1187-1191.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J. Comparison of the tissue receptors for Vibrio cholerae and Escherichia coli enterotoxins by means of gangliosides and natural cholera toxoid. Infect Immun. 1973 Dec;8(6):851–859. doi: 10.1128/iai.8.6.851-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Takeda Y., Miwatani T. Isolation of special antibodies which react only with homologous enterotoxins from Vibrio cholerae and Enterotoxigenic Escherichia coli. Infect Immun. 1981 Nov;34(2):333–336. doi: 10.1128/iai.34.2.333-336.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiwa S. F., Månsson I. Hemagglutinating and hydrophobic surface properties of salmonellae producing enterotoxin neutralized by cholera anti-toxin. Vet Microbiol. 1983 Oct;8(5):443–458. doi: 10.1016/0378-1135(83)90039-1. [DOI] [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. J Biol Chem. 1986 Jun 15;261(17):7906–7911. [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983 Nov;35(1):253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Pearson G. D., Mekalanos J. J. Molecular cloning of Vibrio cholerae enterotoxin genes in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 May;79(9):2976–2980. doi: 10.1073/pnas.79.9.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. W., Hejtmancik K. E., Markel D. E., Craig J. P., Kurosky A. Antigenic specificity of neutralizing antibody to cholera toxin. Infect Immun. 1979 Jun;24(3):774–779. doi: 10.1128/iai.24.3.774-779.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. W., Houston C. W., Koo F. C. Influence of cultural conditions on mitomycin C-mediated bacteriophage induction and release of Salmonella toxin. Infect Immun. 1981 Apr;32(1):232–242. doi: 10.1128/iai.32.1.232-242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. W., Molina N. C., Houston C. W., Fader R. C. Elevated cAMP in intestinal epithelial cells during experimental cholera and salmonellosis. Toxicon. 1983;21(6):761–775. doi: 10.1016/0041-0101(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Peterson J. W., Sandefur P. D. Evidence of a role for permeability factors in the pathogenesis of salmonellosis. Am J Clin Nutr. 1979 Jan;32(1):197–209. doi: 10.1093/ajcn/32.1.197. [DOI] [PubMed] [Google Scholar]
- Sandefur P. D., Peterson J. W. Isolation of skin permeability factors from culture filtrates of Salmonella typhimurium. Infect Immun. 1976 Sep;14(3):671–679. doi: 10.1128/iai.14.3.671-679.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandefur P. D., Peterson J. W. Neutralization of Salmonella toxin-induced elongation of Chinese hamster ovary cells by cholera antitoxin. Infect Immun. 1977 Mar;15(3):988–992. doi: 10.1128/iai.15.3.988-992.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Honda T., Sima H., Tsuji T., Miwatani T. Analysis of antigenic determinants in cholera enterotoxin and heat-labile enterotoxins from human and porcine enterotoxigenic Escherichia coli. Infect Immun. 1983 Jul;41(1):50–53. doi: 10.1128/iai.41.1.50-53.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Nakazawa T., Miyata T., Kaji A., Yokota T. Evolution and structure of two ADP-ribosylation enterotoxins, Escherichia coli heat-labile toxin and cholera toxin. FEBS Lett. 1984 Apr 24;169(2):241–246. doi: 10.1016/0014-5793(84)80326-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Tamura T., Yokota T. Primary structure of heat-labile enterotoxin produced by Escherichia coli pathogenic for humans. J Biol Chem. 1984 Apr 25;259(8):5037–5044. [PubMed] [Google Scholar]