Abstract
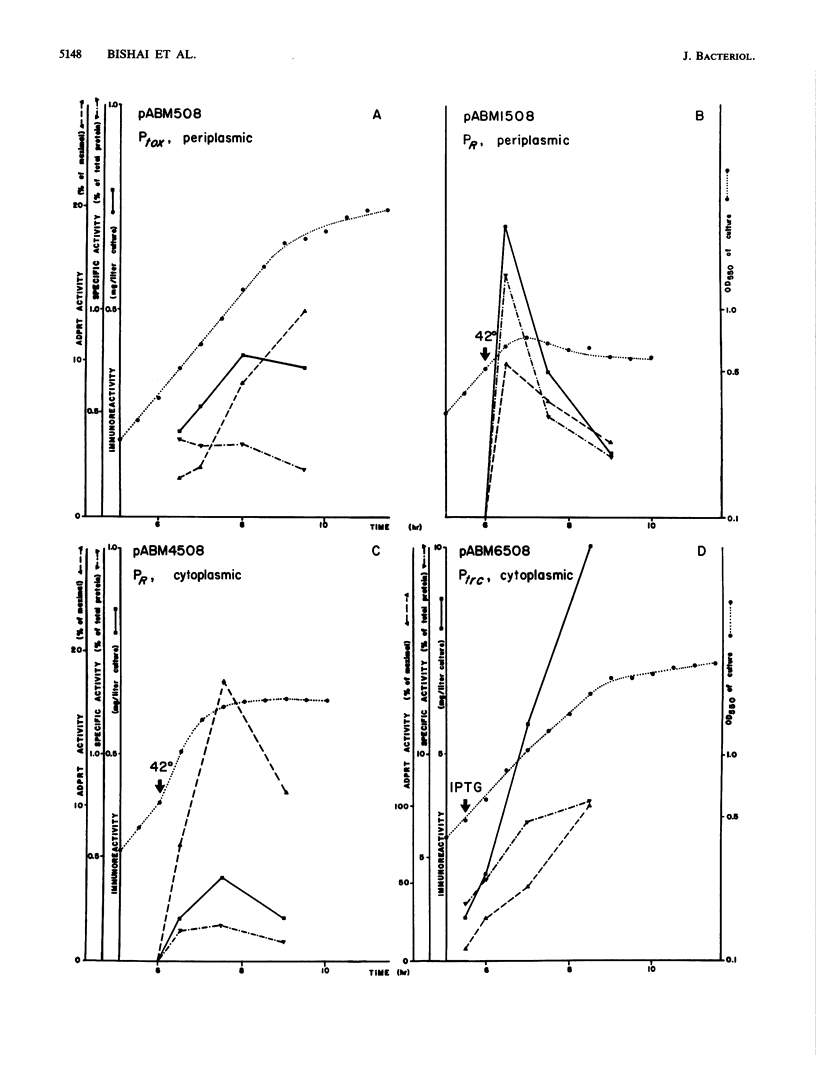
ABM508 is a recombinant fusion protein consisting of the N-terminal 485 amino acids of diphtheria toxin joined to alpha-melanocyte-stimulating hormone. When expressed in Escherichia coli under the control of the tox promoter and signal sequence, ABM508 is severely degraded. When overexpressed from a thermoinducible lambda pR promoter fusion, ABM508 is largely insoluble. We compared the expression of ABM508 (501 amino acids) to a full-length mutant form of the toxin (CRM197; 535 amino acids) and found that CRM197 showed minimal proteolysis. Thus, the removal of the C-terminal 50 amino acids of the toxin destabilizes the protein, making it a target for proteases. Proteolysis of ABM508 could be reduced by removal of the tox signal sequence (thereby directing the protein to the cytoplasm) and growth in lon and htpR mutant strains of E. coli. We also showed that the solubility of tox gene products expressed in E. coli was directly related to the growth temperature of the culture. Thus, a fragment A fusion protein (223 amino acids), ABM508, and CRM197 were found in soluble extracts when expressed at 30 degrees C but could not be released by the same procedures after growth at 42 degrees C. On the basis of these observations, we fused the coding sequences for mature ABM508 to the trc promoter (inducible at 30 degrees C by isopropyl-beta-D-thiogalactoside) and expressed this construct in a lon htpR strain of E. coli. This plasmid made 10 mg of soluble tox protein per liter of culture (7.7% of the total cell protein) or 14 times more than our previous maximal level. Extracts from lon htpR cells harboring this plasmid had high levels of ADP-ribosyltransferase activity, and although proteolysis still occurred, the major tox product corresponded to full-length ABM508.
Full text
PDF

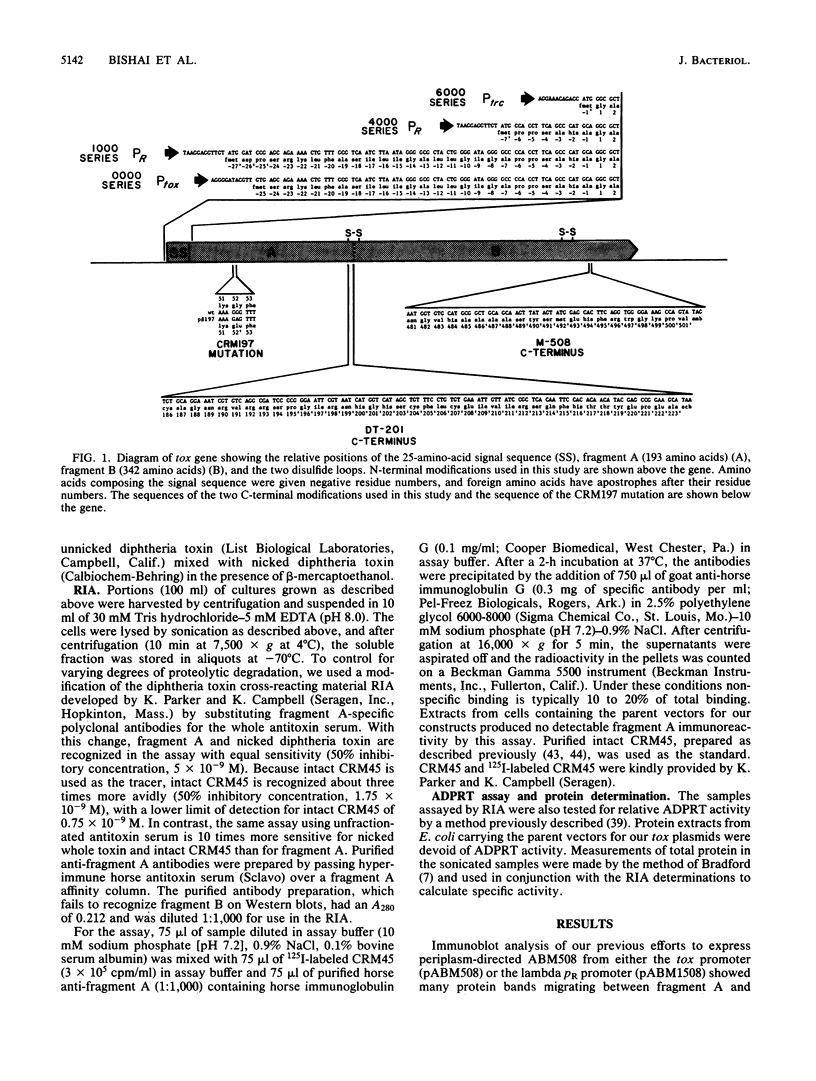








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J. "ATG vectors' for regulated high-level expression of cloned genes in Escherichia coli. Gene. 1985;40(2-3):183–190. doi: 10.1016/0378-1119(85)90041-1. [DOI] [PubMed] [Google Scholar]
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Bacha P., Murphy J. R., Reichlin S. Thyrotropin-releasing hormone-diphtheria toxin-related polypeptide conjugates. Potential role of the hydrophobic domain in toxin entry. J Biol Chem. 1983 Feb 10;258(3):1565–1570. [PubMed] [Google Scholar]
- Baker T. A., Grossman A. D., Gross C. A. A gene regulating the heat shock response in Escherichia coli also affects proteolysis. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6779–6783. doi: 10.1073/pnas.81.21.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S. A., Hall M. N., Silhavy T. J. Genetic analysis of protein export in Escherichia coli K12. Annu Rev Biochem. 1985;54:101–134. doi: 10.1146/annurev.bi.54.070185.000533. [DOI] [PubMed] [Google Scholar]
- Bishai W. R., Miyanohara A., Murphy J. R. Cloning and expression in Escherichia coli of three fragments of diphtheria toxin truncated within fragment B. J Bacteriol. 1987 Apr;169(4):1554–1563. doi: 10.1128/jb.169.4.1554-1563.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. Mechanism of action of the lexA gene product. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4204–4208. doi: 10.1073/pnas.78.7.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Brosius J., Erfle M., Storella J. Spacing of the -10 and -35 regions in the tac promoter. Effect on its in vivo activity. J Biol Chem. 1985 Mar 25;260(6):3539–3541. [PubMed] [Google Scholar]
- Buell G., Schulz M. F., Selzer G., Chollet A., Movva N. R., Semon D., Escanez S., Kawashima E. Optimizing the expression in E. coli of a synthetic gene encoding somatomedin-C (IGF-I). Nucleic Acids Res. 1985 Mar 25;13(6):1923–1938. doi: 10.1093/nar/13.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J. Effect of diphtheria toxin on protein synthesis: inactivation of one of the transfer factors. J Mol Biol. 1967 Apr 14;25(1):83–98. doi: 10.1016/0022-2836(67)90280-x. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan J. J., Simon M. I., Draper R. K., Montal M. Diphtheria toxin forms transmembrane channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jan;78(1):172–176. doi: 10.1073/pnas.78.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazin R., Kandel J., Collier R. J. Structure and activity of diphtheria toxin. II. Attack by trypsin at a specific site within the intact toxin molecule. J Biol Chem. 1971 Mar 10;246(5):1504–1510. [PubMed] [Google Scholar]
- Gentz R., Langner A., Chang A. C., Cohen S. N., Bujard H. Cloning and analysis of strong promoters is made possible by the downstream placement of a RNA termination signal. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4936–4940. doi: 10.1073/pnas.78.8.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini G., Rappuoli R., Ratti G. The amino-acid sequence of two non-toxic mutants of diphtheria toxin: CRM45 and CRM197. Nucleic Acids Res. 1984 May 25;12(10):4063–4069. doi: 10.1093/nar/12.10.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. Bacterial toxins: a table of lethal amounts. Microbiol Rev. 1982 Mar;46(1):86–94. doi: 10.1128/mr.46.1.86-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr Structure-activity relationships in diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1492–1495. [PubMed] [Google Scholar]
- Gilliland D. G., Collier R. J., Moehring J. M., Moehring T. J. Chimeric toxins: toxic, disulfide-linked conjugate of concanavalin A with fragment A from diphtheria toxin. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5319–5323. doi: 10.1073/pnas.75.11.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland D. G., Steplewski Z., Collier R. J., Mitchell K. F., Chang T. H., Koprowski H. Antibody-directed cytotoxic agents: use of monoclonal antibody to direct the action of toxin A chains to colorectal carcinoma cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4539–4543. doi: 10.1073/pnas.77.8.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. A., Casson L. P., Goldberg A. L. Heat shock regulatory gene htpR influences rates of protein degradation and expression of the lon gene in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6647–6651. doi: 10.1073/pnas.81.21.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goor R. S., Pappenheimer A. M., Jr Studies on the mode of action of diphtheria toxin. 3. Site of toxin action in cell-free extracts. J Exp Med. 1967 Nov 1;126(5):899–912. doi: 10.1084/jem.126.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield L., Bjorn M. J., Horn G., Fong D., Buck G. A., Collier R. J., Kaplan D. A. Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage beta. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6853–6857. doi: 10.1073/pnas.80.22.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A. D., Erickson J. W., Gross C. A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984 Sep;38(2):383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Lazaar A. L., Syvanen M. Regulation of Tn5 by the right-repeat proteins: control at the level of the transposition reaction? Cell. 1982 Oct;30(3):883–892. doi: 10.1016/0092-8674(82)90293-8. [DOI] [PubMed] [Google Scholar]
- Ittelson T. R., Gill D. M. Diphtheria toxin: specific competition for cell receptors. Nature. 1973 Mar 30;242(5396):330–332. doi: 10.1038/242330b0. [DOI] [PubMed] [Google Scholar]
- Kaczorek M., Delpeyroux F., Chenciner N., Streeck R. E., Murphy J. R., Boquet P., Tiollais P. Nucleotide sequence and expression of the diphtheria tox228 gene in Escherichia coli. Science. 1983 Aug 26;221(4613):855–858. doi: 10.1126/science.6348945. [DOI] [PubMed] [Google Scholar]
- Kaczorek M., Zettlmeissl G., Delpeyroux F., Streeck R. E. Diphtheria toxin promoter function in Corynebacterium diphtheriae and Escherichia coli. Nucleic Acids Res. 1985 May 10;13(9):3147–3159. doi: 10.1093/nar/13.9.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong D., Coleman K. D., Murphy J. R. Cloned fragment A of diphtheria toxin is expressed and secreted into the periplasmic space of Escherichia coli K12. Science. 1983 Apr 29;220(4596):515–517. doi: 10.1126/science.6403984. [DOI] [PubMed] [Google Scholar]
- Marnell M. H., Shia S. P., Stookey M., Draper R. K. Evidence for penetration of diphtheria toxin to the cytosol through a prelysosomal membrane. Infect Immun. 1984 Apr;44(1):145–150. doi: 10.1128/iai.44.1.145-150.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Bishai W., Borowski M., Miyanohara A., Boyd J., Nagle S. Genetic construction, expression, and melanoma-selective cytotoxicity of a diphtheria toxin-related alpha-melanocyte-stimulating hormone fusion protein. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8258–8262. doi: 10.1073/pnas.83.21.8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Pakula A. A., Young V. B., Sauer R. T. Bacteriophage lambda cro mutations: effects on activity and intracellular degradation. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8829–8833. doi: 10.1073/pnas.83.23.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Ratti G., Rappuoli R., Giannini G. The complete nucleotide sequence of the gene coding for diphtheria toxin in the corynephage omega (tox+) genome. Nucleic Acids Res. 1983 Oct 11;11(19):6589–6595. doi: 10.1093/nar/11.19.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righelato R. C., van Hemert P. A. Growth and toxin synthesis in batch and chemostat cultures of Corynebacterium diphtheriae. J Gen Microbiol. 1969 Nov;58(3):403–410. doi: 10.1099/00221287-58-3-403. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Rapid entry of nicked diphtheria toxin into cells at low pH. Characterization of the entry process and effects of low pH on the toxin molecule. J Biol Chem. 1981 Sep 10;256(17):9068–9076. [PubMed] [Google Scholar]
- Straus D., Gilbert W. Chicken triosephosphate isomerase complements an Escherichia coli deficiency. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2014–2018. doi: 10.1073/pnas.82.7.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy K. H., Goldberg A. L. Subcellular distribution of various proteases in Escherichia coli. J Bacteriol. 1982 Mar;149(3):1027–1033. doi: 10.1128/jb.149.3.1027-1033.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K., Gilbert W. Cellular location affects protein stability in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1830–1833. doi: 10.1073/pnas.79.6.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe P. E., Ross W. C., Cumber A. J., Hinson C. A., Edwards D. C., Davies A. J. Toxicity of diphtheria toxin for lymphoblastoid cells is increased by conjugation to antilymphocytic globulin. Nature. 1978 Feb 23;271(5647):752–755. doi: 10.1038/271752a0. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Tweten R. K., Collier R. J. Molecular cloning and expression of gene fragments from corynebacteriophage beta encoding enzymatically active peptides of diphtheria toxin. J Bacteriol. 1983 Nov;156(2):680–685. doi: 10.1128/jb.156.2.680-685.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Greany R. Diphtheria toxin and related proteins. I. Isolation and properties of mutant proteins serologically related to diphtheria toxin. J Biol Chem. 1973 Jun 10;248(11):3838–3844. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wood C. R., Boss M. A., Patel T. P., Emtage J. S. The influence of messenger RNA secondary structure on expression of an immunoglobulin heavy chain in Escherichia coli. Nucleic Acids Res. 1984 May 11;12(9):3937–3950. doi: 10.1093/nar/12.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaizumi M., Uchida T., Takamatsu K., Okada Y. Intracellular stability of diphtheria toxin fragment A in the presence and absence of anti-fragment A antibody. Proc Natl Acad Sci U S A. 1982 Jan;79(2):461–465. doi: 10.1073/pnas.79.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zettlmeissl G., Kaczorek M., Moya M., Streeck R. E. Expression of immunogenically reactive diphtheria toxin fusion proteins under the control of the pR promoter of bacteriophage lambda. Gene. 1986;41(1):103–111. doi: 10.1016/0378-1119(86)90272-6. [DOI] [PubMed] [Google Scholar]
- Zhao J. M., London E. Similarity of the conformation of diphtheria toxin at high temperature to that in the membrane-penetrating low-pH state. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2002–2006. doi: 10.1073/pnas.83.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]