Abstract
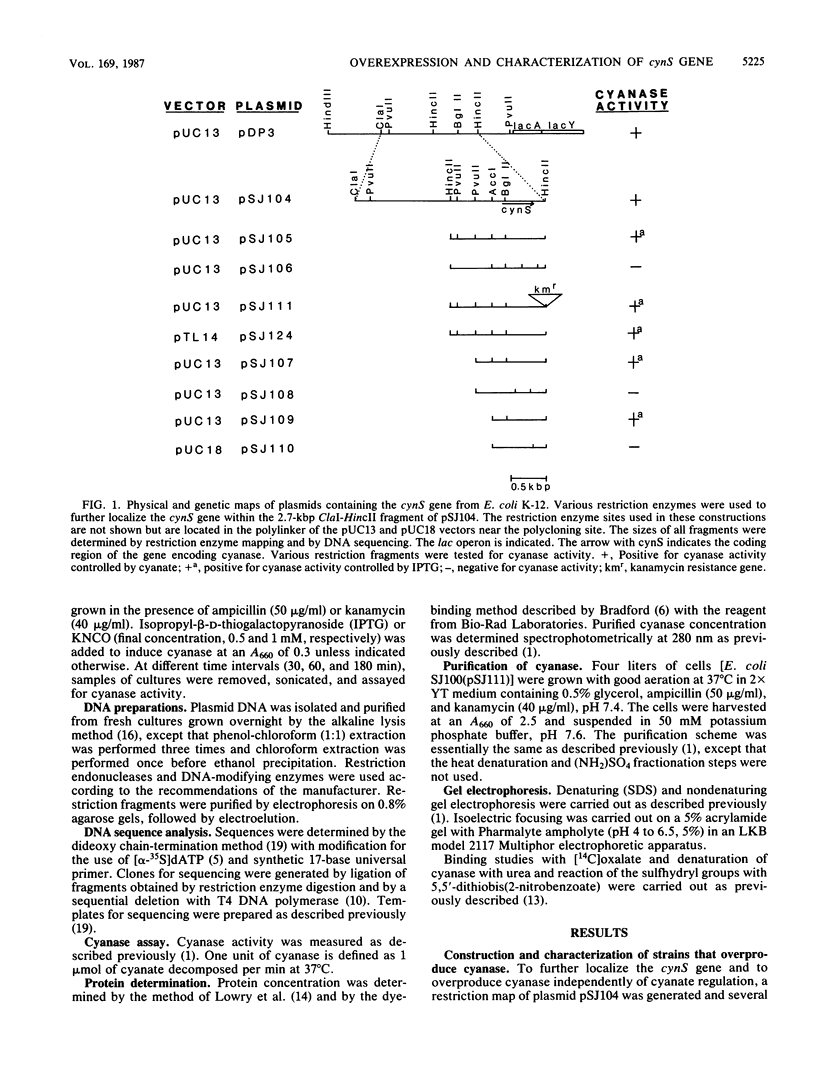
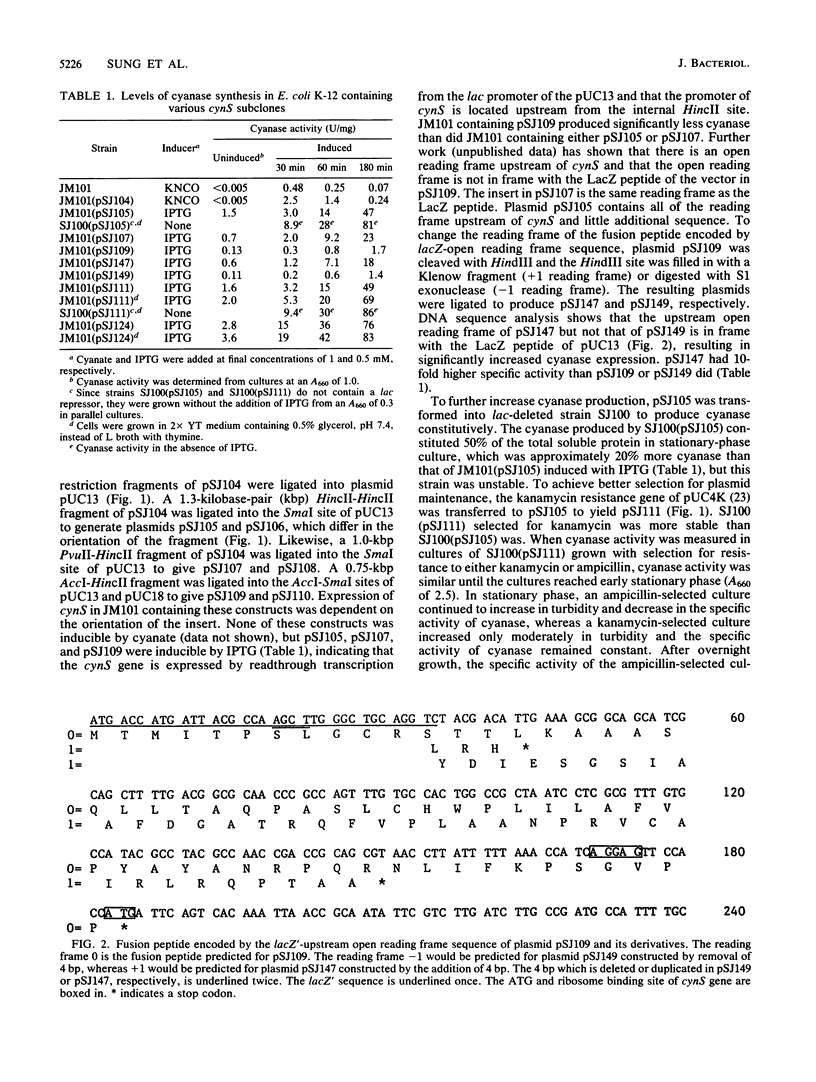
Restriction fragments containing the gene encoding cyanase, cynS, without its transcriptional regulatory sequences were placed downstream of lac and tac promoters in various pUC derivatives to maximize production of cyanase. Plasmid pSJ105, which contains the cynS gene and an upstream open reading frame, gave the highest expression of cyanase. Approximately 50% of the total soluble protein in stationary-phase cultures of a lac-deleted strain containing plasmid pSJ105 was cyanase. The inserted DNA fragment of pSJ105 was transferred into pUC18 derivatives that contain a hybrid tac promoter, instead of the lac promoter, and a strong terminator to generate pSJ124. Stationary-phase cultures of JM101 containing plasmid pSJ124 overexpressed a similar level of cyanase. In JM101(pSJ124), maximum production of cyanase could be obtained either by induction with isopropyl-beta-D-thiogalactopyranoside (IPTG) for 3 h or by growth without IPTG into late stationary phase. The latter conditions resulted in a 10- to 20-fold increase in plasmid content and presumably titration of the lac repressor. The nucleotide sequence of the cloned cynS gene from Escherichia coli K-12 was determined. The predicted amino acid sequence differed from the known amino acid sequence of cyanase isolated from a B strain by four residues. However, overexpressed cyanase was purified to homogeneity, and a comparison of the enzymes from the two sources indicated that they did not differ with respect to physical and kinetic properties. The cynS gene was located next to the lac operon, and the direction of cynS transcription was opposite that of lac.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Johnson W. V., Endrizzi J. A., Little R. M., Korte J. J. Interaction of mono- and dianions with cyanase: evidence for apparent half-site binding. Biochemistry. 1987 Jun 30;26(13):3938–3943. doi: 10.1021/bi00387a029. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Little R. M. Kinetic properties of cyanase. Biochemistry. 1986 Apr 8;25(7):1621–1626. doi: 10.1021/bi00355a026. [DOI] [PubMed] [Google Scholar]
- Anderson P. M. Purification and properties of the inducible enzyme cyanase. Biochemistry. 1980 Jun 24;19(13):2882–2888. doi: 10.1021/bi00554a010. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Brosius J., Ullrich A., Raker M. A., Gray A., Dull T. J., Gutell R. R., Noller H. F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981 Jul;6(1):112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Chin C. C., Anderson P. M., Wold F. The amino acid sequence of Escherichia coli cyanase. J Biol Chem. 1983 Jan 10;258(1):276–282. [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Guilloton M., Karst F. Isolation and characterization of Escherichia coli mutants lacking inducible cyanase. J Gen Microbiol. 1987 Mar;133(3):645–653. doi: 10.1099/00221287-133-3-645. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Little R. M., Anderson P. M. Structural properties of cyanase. Denaturation, renaturation, and role of sulfhydryls and oligomeric structure in catalytic activity. J Biol Chem. 1987 Jul 25;262(21):10120–10126. [PubMed] [Google Scholar]
- Lunn C. A., Kathju S., Wallace B. J., Kushner S. R., Pigiet V. Amplification and purification of plasmid-encoded thioredoxin from Escherichia coli K12. J Biol Chem. 1984 Aug 25;259(16):10469–10474. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sung Y. C., Parsell D., Anderson P. M., Fuchs J. A. Identification, mapping, and cloning of the gene encoding cyanase in Escherichia coli K-12. J Bacteriol. 1987 Jun;169(6):2639–2642. doi: 10.1128/jb.169.6.2639-2642.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUSSIG A. The synthesis of the induced enzyme, ''cyanase'', in E. coli. Biochim Biophys Acta. 1960 Nov 18;44:510–519. doi: 10.1016/0006-3002(60)91605-x. [DOI] [PubMed] [Google Scholar]
- Taussig A. Some properties of the induced enzyme cyanase. Can J Biochem. 1965 Jul;43(7):1063–1069. doi: 10.1139/o65-119. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]