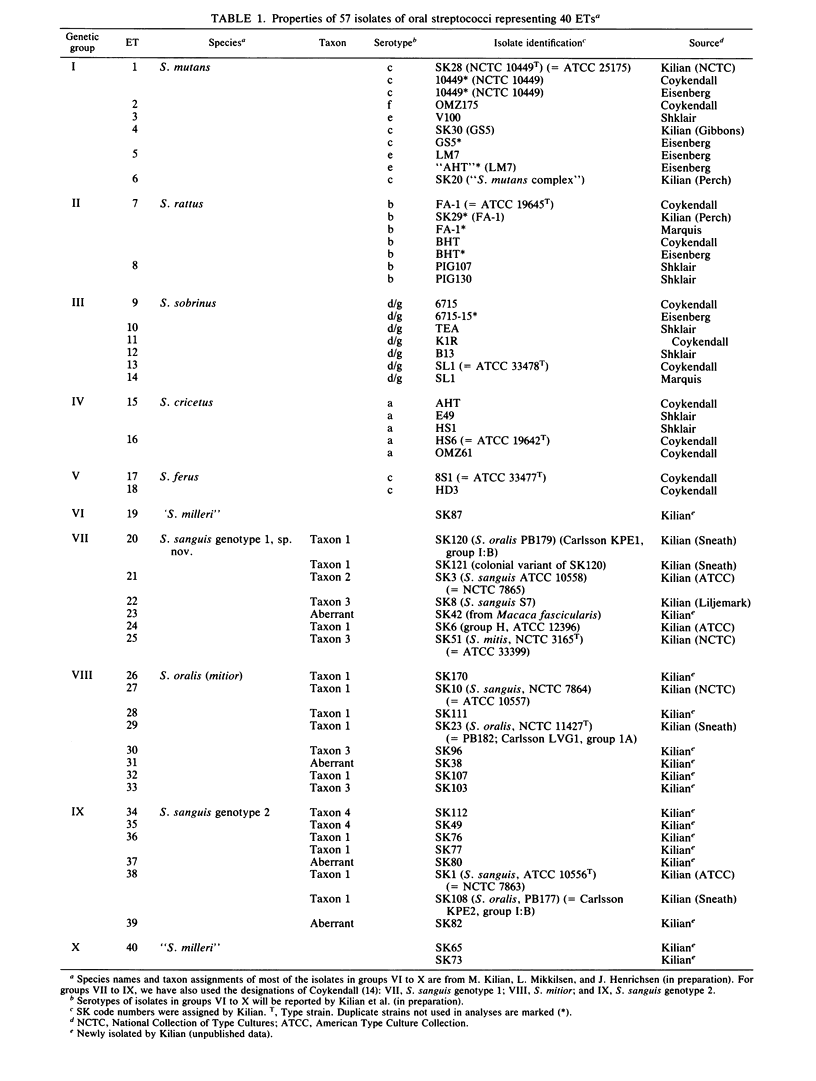
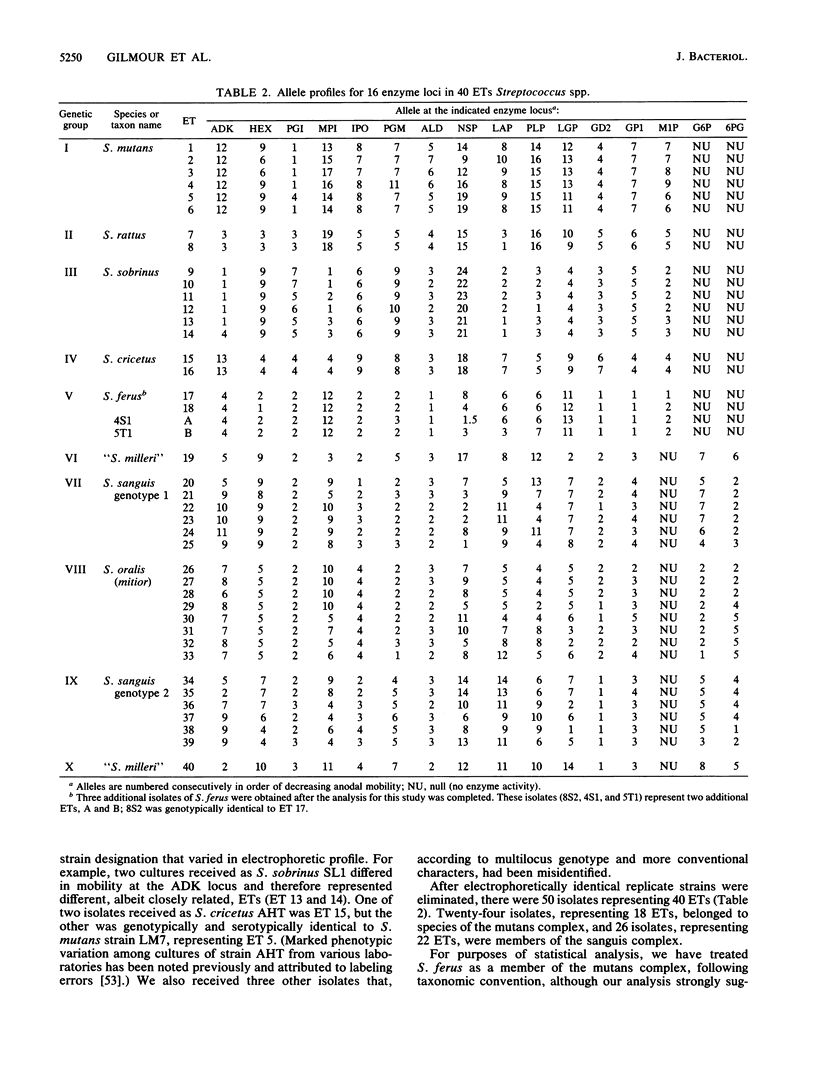
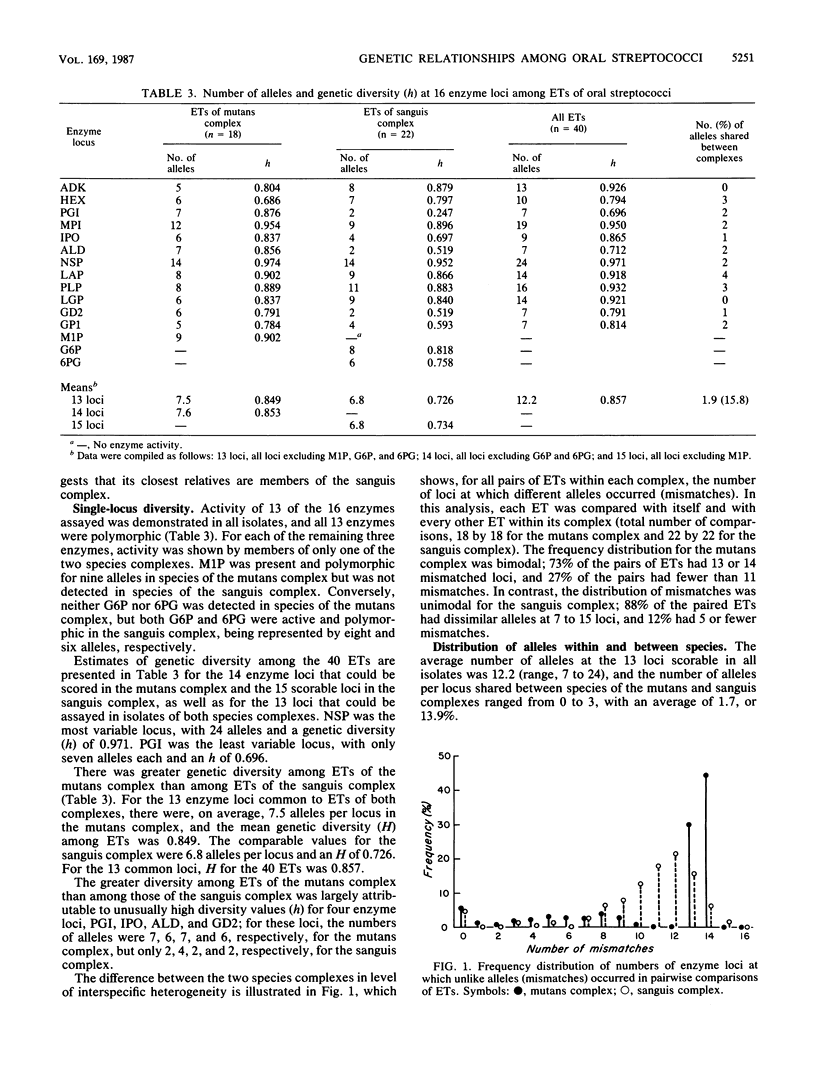
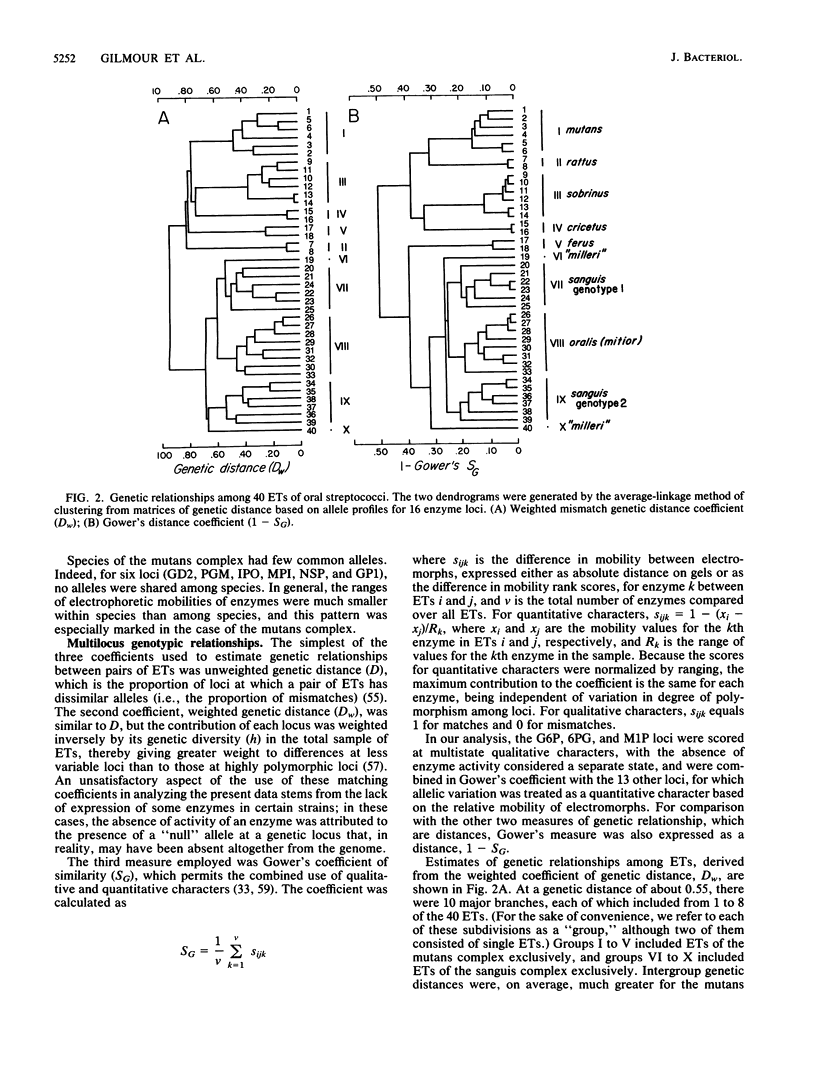
Abstract
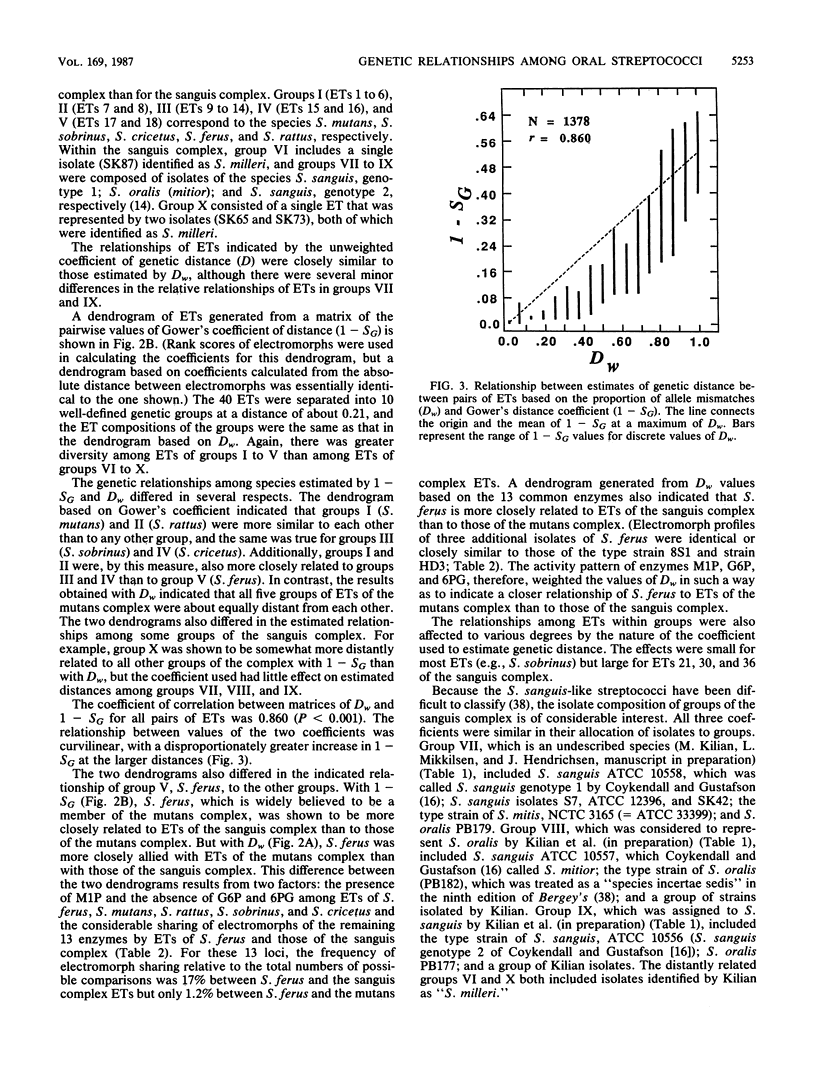
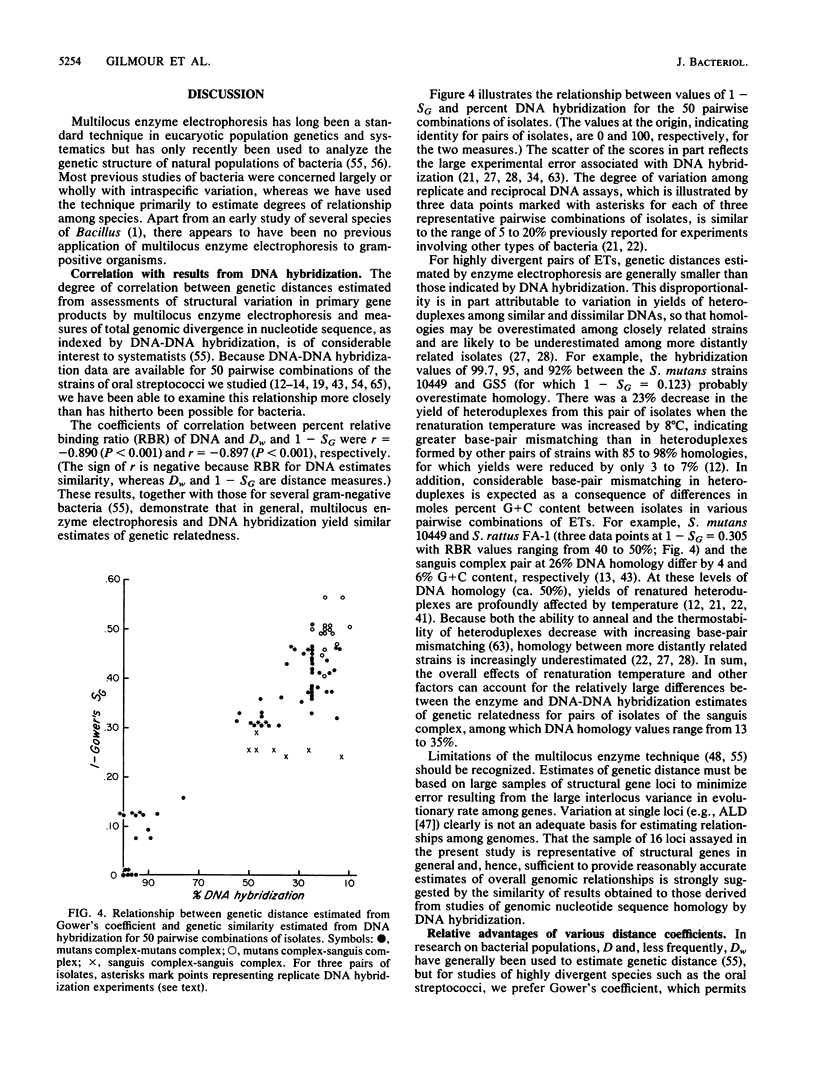
Genetic relationships and species limits among the oral streptococci were determined by an analysis of electrophoretically demonstrable variation in 16 metabolic enzymes. Fifty isolates represented 40 electrophoretic types, among which the mean genetic diversity per locus was 0.857. Mannitol-1-phosphate dehydrogenase was not detected in isolates of the sanguis species complex, and glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase were absent in species of the mutans complex. Clustering from a matrix of Gower's coefficient of genetic similarity placed the 40 electrophoretic types in 10 well-defined groups corresponding to the Streptococcus species S. mutans, S. sobrinus, S. cricetus, S. rattus, S. ferus, S. oralis (mitior), two distinct assemblages of S. sanguis strains, and two subdivisions of "S. milleri." The assignments of isolates to these groups were the same as those indicated by DNA hybridization experiments, and the coefficient of correlation between genetic distance estimated by multilocus enzyme electrophoresis and genetic similarity indexed by DNA hybridization was -0.897 (P less than 0.001) for 50 pairwise combinations of isolates. S. ferus, which is widely believed to be a member of the mutans complex, was shown to be phylogenetically closer to species of the sanguis complex.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyar R. M., Bowden G. H. The microflora associated with the progression of incipient carious lesions of children living in a water-fluoridated area. Caries Res. 1985;19(4):298–306. doi: 10.1159/000260859. [DOI] [PubMed] [Google Scholar]
- Bridge P. D., Sneath P. H. Numerical taxonomy of Streptococcus. J Gen Microbiol. 1983 Mar;129(3):565–597. doi: 10.1099/00221287-129-3-565. [DOI] [PubMed] [Google Scholar]
- Bridges R. B. Ribose biosynthesis in Streptococcus mutans. Arch Oral Biol. 1977;22(2):139–145. doi: 10.1016/0003-9969(77)90091-7. [DOI] [PubMed] [Google Scholar]
- Brown A. T., Patterson C. E. Heterogeneity of Streptococcus mutans strains based on their mannitol-1-phosphate dehydrogenases: criterion for rapid classification. Infect Immun. 1972 Sep;6(3):422–424. doi: 10.1128/iai.6.3.422-424.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Frøholm L. O., Bøvre K., Holten E., Frasch C. E., Mocca L. F., Zollinger W. D., Selander R. K. Intercontinental spread of a genetically distinctive complex of clones of Neisseria meningitidis causing epidemic disease. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4927–4931. doi: 10.1073/pnas.83.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986;40:635–659. doi: 10.1146/annurev.mi.40.100186.003223. [DOI] [PubMed] [Google Scholar]
- Coakley W. T., Bater A. J., Lloyd D. Disruption of micro-organisms. Adv Microb Physiol. 1977;16:279–341. doi: 10.1016/s0065-2911(08)60050-8. [DOI] [PubMed] [Google Scholar]
- Coykendall A. L., Bratthall D., O'Connor K., Dvarskas R. A. Serological and genetic examination of some nontypical Streptococcus mutans strains. Infect Immun. 1976 Sep;14(3):667–670. doi: 10.1128/iai.14.3.667-670.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coykendall A. L. Four types of Streptococcus mutans based on their genetic, antigenic and biochemical characteristics. J Gen Microbiol. 1974 Aug;83(2):327–338. doi: 10.1099/00221287-83-2-327. [DOI] [PubMed] [Google Scholar]
- Coykendall A. L. Genetic heterogeneity in Streptococcus mutans. J Bacteriol. 1971 Apr;106(1):192–196. doi: 10.1128/jb.106.1.192-196.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coykendall A. L., Specht P. A. DNA base sequence homologies among strains of Streptococcus sanguis. J Gen Microbiol. 1975 Nov;91(1):92–98. doi: 10.1099/00221287-91-1-92. [DOI] [PubMed] [Google Scholar]
- Coykendall A. L., Specht P. A., Samol H. H. Streptococcus mutans in a wild, sucrose-eating rat population. Infect Immun. 1974 Jul;10(1):216–219. doi: 10.1128/iai.10.1.216-219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I., Russell C. Streptococci isolated from the bloodstream and gingival crevice of man. J Med Microbiol. 1983 Aug;16(3):263–269. doi: 10.1099/00222615-16-3-263. [DOI] [PubMed] [Google Scholar]
- De Ley J., Tijtgat R. Evaluation of membrane filter methods for DNA-DNA hybridization. Antonie Van Leeuwenhoek. 1970;36(4):461–474. doi: 10.1007/BF02069048. [DOI] [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. A., Birfelder E. J., Sanders J. P., Borst P. DNA-DNA hybridization on nitrocellulose filters. 1. General considerations and non-ideal kinetics. Eur J Biochem. 1974 Sep 16;47(3):535–543. doi: 10.1111/j.1432-1033.1974.tb03722.x. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Borst P., Birfelder E. J. DNA-DNA hybridization on nitrocellulose filters. 2. Concatenation effects. Eur J Biochem. 1974 Sep 16;47(3):545–548. doi: 10.1111/j.1432-1033.1974.tb03723.x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Cohen L., Hay D. I. Strains of Streptococcus mutans and Streptococcus sobrinus attach to different pellicle receptors. Infect Immun. 1986 May;52(2):555–561. doi: 10.1128/iai.52.2.555-561.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Virulence-related physiological changes and antigenic variation in populations of Streptococcus mutans colonizing gnotobiotic rats. Infect Immun. 1980 Sep;29(3):1082–1091. doi: 10.1128/iai.29.3.1082-1091.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Lo G. C. Co-induction of beta-galactosidase and the lactose-P-enolpyruvate phosphotransferase system in Streptococcus salivarius and Streptococcus mutans. J Bacteriol. 1978 Dec;136(3):900–908. doi: 10.1128/jb.136.3.900-908.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper D. S., Loesche W. J. Growth and acid tolerance of human dental plaque bacteria. Arch Oral Biol. 1984;29(10):843–848. doi: 10.1016/0003-9969(84)90015-3. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Ordal E. J. Deoxyribonucleic acid homology in bacterial taxonomy: effect of incubation temperature on reaction specificity. J Bacteriol. 1968 Mar;95(3):893–900. doi: 10.1128/jb.95.3.893-900.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. V., Maier S. Correction for the inherent error in optical density readings. Appl Environ Microbiol. 1977 Feb;33(2):482–484. doi: 10.1128/aem.33.2.482-484.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Barenkamp S. J., Granoff D. M., Selander R. K. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect Immun. 1986 Apr;52(1):183–191. doi: 10.1128/iai.52.1.183-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Hewlett E. L., Peppler M. S., Selander R. K. Genetic diversity and relationships in populations of Bordetella spp. J Bacteriol. 1986 Apr;166(1):230–237. doi: 10.1128/jb.166.1.230-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. T., Ball L. C. Streptococci and aerococci associated with systemic infection in man. J Med Microbiol. 1976 Aug;9(3):275–302. doi: 10.1099/00222615-9-3-275. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Comparison of oral Streptococcus mutans AHT with strains of serotypes a and g by biochemical and electrophoretic methods. Arch Oral Biol. 1979;24(8):617–619. doi: 10.1016/0003-9969(79)90023-2. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kilpper-Bälz R., Kraus J., Gehring F. Relatedness and classification of Streptococcus mutans and "mutans-like" streptococci. J Dent Res. 1984 Aug;63(8):1047–1050. doi: 10.1177/00220345840630080701. [DOI] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., McKinney R. M., Whittam T. S., Bibb W. F., Brenner D. J., Nolte F. S., Pattison P. E. Genetic structure of populations of Legionella pneumophila. J Bacteriol. 1985 Sep;163(3):1021–1037. doi: 10.1128/jb.163.3.1021-1037.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Brown A. T., McInerney M. F., Woodiel F. N. Comparative study of invertases of Streptococcus mutans. Infect Immun. 1977 Apr;16(1):318–327. doi: 10.1128/iai.16.1.318-327.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilade E., Theilade J., Mikkelsen L. Microbiological studies on early dento-gingival plaque on teeth and Mylar strips in humans. J Periodontal Res. 1982 Jan;17(1):12–25. doi: 10.1111/j.1600-0765.1982.tb01127.x. [DOI] [PubMed] [Google Scholar]
- Tsumori H., Shimamura A., Mukasa H. Comparative study of Streptococcus mutans extracellular glycosyltransferases by isoelectric focusing. J Gen Microbiol. 1983 Oct;129(10):3261–3269. doi: 10.1099/00221287-129-10-3261. [DOI] [PubMed] [Google Scholar]
- Ullman J. S., McCarthy B. J. The relationship between mismatched base pairs and the thermal stability of DNA duplexes. II. Effects of deamination of cytosine. Biochim Biophys Acta. 1973 Feb 4;294(1):416–424. doi: 10.1016/0005-2787(73)90096-8. [DOI] [PubMed] [Google Scholar]
- Westergren G., Emilson C. G. Prevalence of transformable Streptococcus mutans in human dental plaque. Infect Immun. 1983 Sep;41(3):1386–1388. doi: 10.1128/iai.41.3.1386-1388.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergren G., Emilson C. G. Transformation of streptococci to streptomycin resistance by oral streptococcal DNA. Arch Oral Biol. 1977;22(8-9):533–537. doi: 10.1016/0003-9969(77)90051-6. [DOI] [PubMed] [Google Scholar]
- van Houte J., Russo J. Variable colonization by oral streptococci in molar fissures of monoinfected gnotobiotic rats. Infect Immun. 1986 May;52(2):620–622. doi: 10.1128/iai.52.2.620-622.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


