Abstract
Escherichia coli mutants simultaneously resistant to rifampin and to the lethal effects of bacteriophage lambda cII protein were isolated. The sck mutant strains carry alterations in rpoB that allow them to survive cII killing (thus the name sck), but that do not impair either the expression of cII or the activation by cII of the lambda promoters pE and pI. The sck-1, sck-2, and sck-3 mutations modify transcription termination. The growth of lambda, but not of the N-independent lambda variant, lambda nin-5, is hindered by these mutations, which act either alone or in concert with the bacterial nusA1 mutation. In contrast to their effect on lambda growth, the three mutations reduce transcription termination in bacterial operons. The E. coli pyrE gene, which is normally regulated by attenuation, is expressed constitutively in the mutant strains. The sck mutations appear to prevent pyrE attenuation by slowing the rate of transcriptional elongation of the pyrE leader sequence. The sck-6 mutation, unlike the other sck mutations, neither increases pyrE expression nor inhibits the ability of lambda to suppress transcription termination. Instead, the sck-6 mutation blocks the growth of the lambda variants lambda nin-5 and lambda red-3.
Full text
PDF
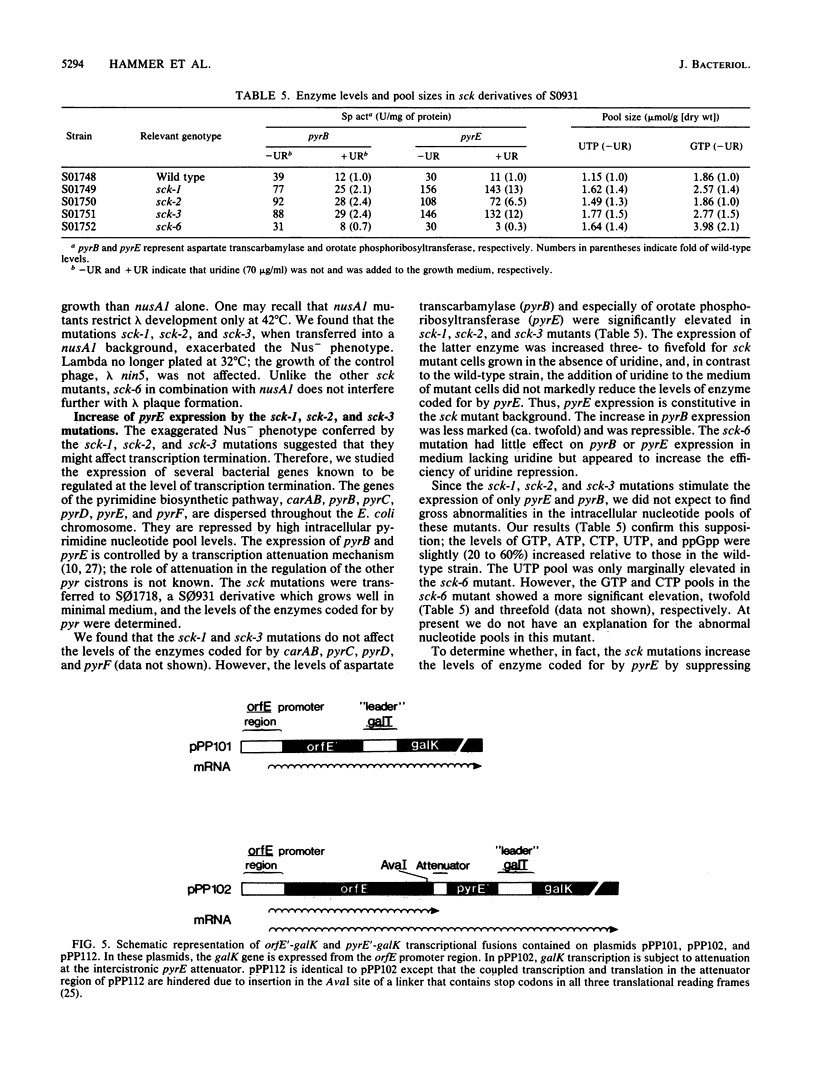
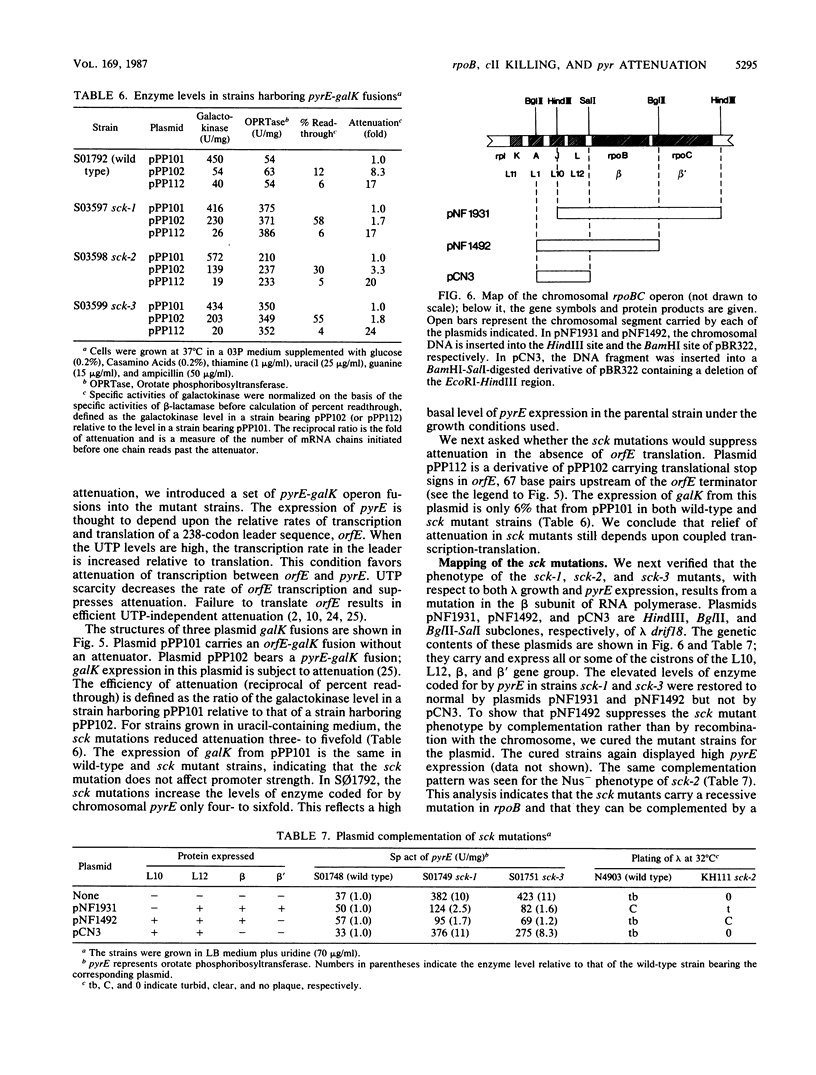


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bonekamp F., Clemmesen K., Karlström O., Jensen K. F. Mechanism of UTP-modulated attenuation at the pyrE gene of Escherichia coli: an example of operon polarity control through the coupling of translation to transcription. EMBO J. 1984 Dec 1;3(12):2857–2861. doi: 10.1002/j.1460-2075.1984.tb02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buxton R. S., Albrechtsen H., Hammer-Jespersen K. Overlapping transcriptional units in the deo operon of Escherichia coli K-12. Evidence from phage Mu-1 insertion mutants. J Mol Biol. 1977 Aug 15;114(3):287–300. doi: 10.1016/0022-2836(77)90251-0. [DOI] [PubMed] [Google Scholar]
- Dandanell G., Hammer K. Two operator sites separated by 599 base pairs are required for deoR repression of the deo operon of Escherichia coli. EMBO J. 1985 Dec 1;4(12):3333–3338. doi: 10.1002/j.1460-2075.1985.tb04085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin G., Maaloe O. Synthesis and breakdown of messenger RNA without protein synthesis. J Mol Biol. 1966 Feb;15(2):428–434. doi: 10.1016/s0022-2836(66)80118-3. [DOI] [PubMed] [Google Scholar]
- Fiil N. P., Bendiak D., Collins J., Friesen J. D. Expression of Escherichia coli ribosomal protein and RNA polymerase genes cloned on plasmids. Mol Gen Genet. 1979 May 23;173(1):39–50. doi: 10.1007/BF00267689. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. R., Georgopoulos C., Tilly K., Herskowitz I., Banuett F. Interactions of bacteriophage and host macromolecules in the growth of bacteriophage lambda. Microbiol Rev. 1984 Dec;48(4):299–325. doi: 10.1128/mr.48.4.299-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes B. C., McClure W. R. A cII-dependent promoter is located within the Q gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1985 May;82(10):3134–3138. doi: 10.1073/pnas.82.10.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. F., Fast R., Karlström O., Larsen J. N. Association of RNA polymerase having increased Km for ATP and UTP with hyperexpression of the pyrB and pyrE genes of Salmonella typhimurium. J Bacteriol. 1986 Jun;166(3):857–865. doi: 10.1128/jb.166.3.857-865.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. F., Houlberg U., Nygaard P. Thin-layer chromatographic methods to isolate 32P-labeled 5-phosphoribosyl-alpha-1-pyrophosphate (PRPP): determination of cellular PRPP pools and assay of PRPP synthetase activity. Anal Biochem. 1979 Oct 1;98(2):254–263. doi: 10.1016/0003-2697(79)90138-6. [DOI] [PubMed] [Google Scholar]
- Jensen K. F., Larsen J. N., Schack L., Sivertsen A. Studies on the structure and expression of Escherichia coli pyrC, pyrD, and pyrF using the cloned genes. Eur J Biochem. 1984 Apr 16;140(2):343–352. doi: 10.1111/j.1432-1033.1984.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Jensen K. F., Neuhard J., Schack L. RNA polymerase involvement in the regulation of expression of Salmonella typhimurium pyr genes. Isolation and characterization of a fluorouracil-resistant mutant with high, constitutive expression of the pyrB and pyrE genes due to a mutation in rpoBC. EMBO J. 1982;1(1):69–74. doi: 10.1002/j.1460-2075.1982.tb01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum J. B., Konrad E. B. Isolation of a specialized lambda transducing bacteriophage carrying the beta subunit gene for Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1973 Nov;116(2):517–526. doi: 10.1128/jb.116.2.517-526.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahajna J., Oppenheim A. B., Rattray A., Gottesman M. Translation initiation of bacteriophage lambda gene cII requires integration host factor. J Bacteriol. 1986 Jan;165(1):167–174. doi: 10.1128/jb.165.1.167-174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim A. B., Gottesman S., Gottesman M. Regulation of bacteriophage lambda int gene expression. J Mol Biol. 1982 Jul 5;158(3):327–346. doi: 10.1016/0022-2836(82)90201-7. [DOI] [PubMed] [Google Scholar]
- Oppenheim A. B., Mahajna G., Koby S., Altuvia S. Regulation of the establishment of repressor synthesis in bacteriophage lambda. J Mol Biol. 1982 Feb 25;155(2):121–132. doi: 10.1016/0022-2836(82)90440-5. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Monastyrskaya G. S., Guriev S. O., Kalinina N. F., Sverdlov E. D., Gragerov A. I., Bass I. A., Kiver I. F., Moiseyeva E. P., Igumnov V. N. RNA polymerase rifampicin resistance mutations in Escherichia coli: sequence changes and dominance. Mol Gen Genet. 1983;190(2):344–348. doi: 10.1007/BF00330662. [DOI] [PubMed] [Google Scholar]
- Poulsen P., Bonekamp F., Jensen K. F. Structure of the Escherichia coli pyrE operon and control of pyrE expression by a UTP modulated intercistronic attentuation. EMBO J. 1984 Aug;3(8):1783–1790. doi: 10.1002/j.1460-2075.1984.tb02046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen P., Jensen K. F. Effect of UTP and GTP pools on attenuation at the pyrE gene of Escherichia coli. Mol Gen Genet. 1987 Jun;208(1-2):152–158. doi: 10.1007/BF00330436. [DOI] [PubMed] [Google Scholar]
- Rattray A., Altuvia S., Mahajna G., Oppenheim A. B., Gottesman M. Control of bacteriophage lambda CII activity by bacteriophage and host functions. J Bacteriol. 1984 Jul;159(1):238–242. doi: 10.1128/jb.159.1.238-242.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland K. L., Powell F. E., Turnbough C. L., Jr Role of translation and attenuation in the control of pyrBI operon expression in Escherichia coli K-12. J Bacteriol. 1985 Sep;163(3):991–999. doi: 10.1128/jb.163.3.991-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simatake H., Rosenberg M. Purified lambda regulatory protein cII positively activates promoters for lysogenic development. Nature. 1981 Jul 9;292(5819):128–132. doi: 10.1038/292128a0. [DOI] [PubMed] [Google Scholar]
- Toothman P., Herskowitz I. Rex-dependent exclusion of lambdoid phages. I. Prophage requirements for exclusion. Virology. 1980 Apr 15;102(1):133–146. doi: 10.1016/0042-6822(80)90076-8. [DOI] [PubMed] [Google Scholar]
- Trisler P., Gottesman S. lon transcriptional regulation of genes necessary for capsular polysaccharide synthesis in Escherichia coli K-12. J Bacteriol. 1984 Oct;160(1):184–191. doi: 10.1128/jb.160.1.184-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Svenningsen B. A., Munch-Petersen A., Hammer-Jespersen K. Regulation of the deo operon in Escherichia coli: the double negative control of the deo operon by the cytR and deoR repressors in a DNA directed in vitro system. Mol Gen Genet. 1978 Feb 16;159(2):191–202. doi: 10.1007/BF00270893. [DOI] [PubMed] [Google Scholar]
- Warren F., Das A. Formation of termination-resistant transcription complex at phage lambda nut locus: effects of altered translation and a ribosomal mutation. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3612–3616. doi: 10.1073/pnas.81.12.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]


