Abstract
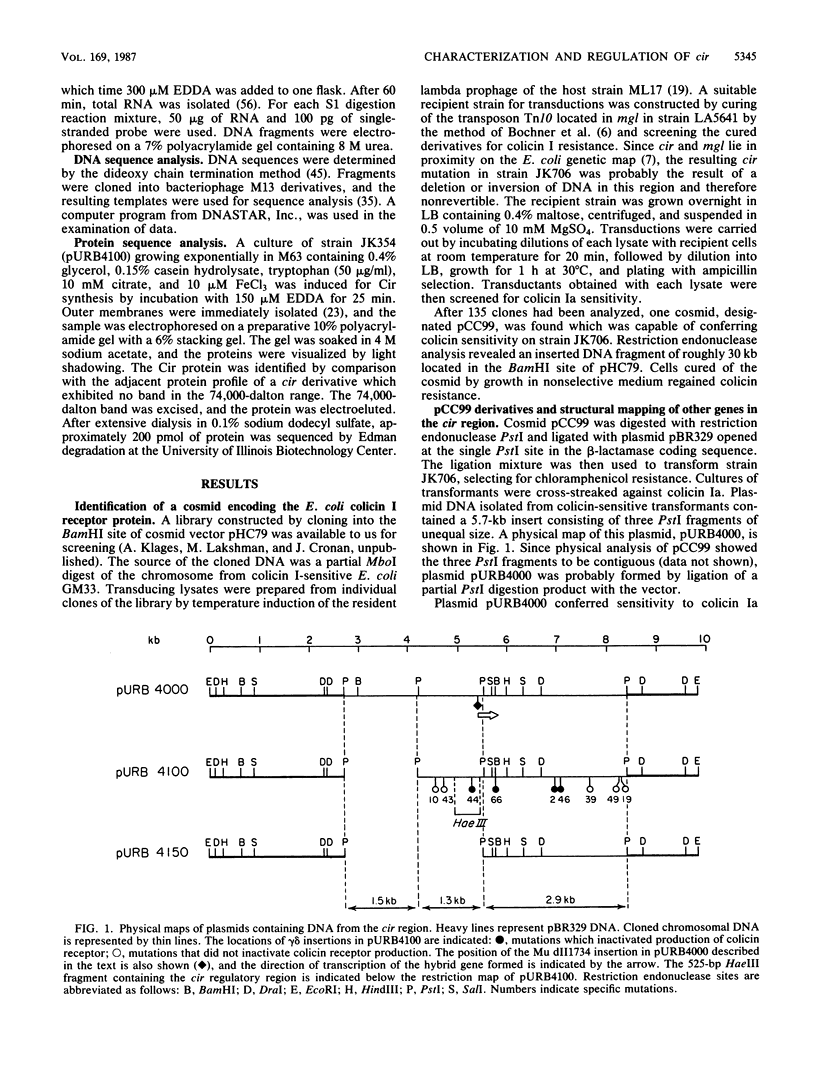
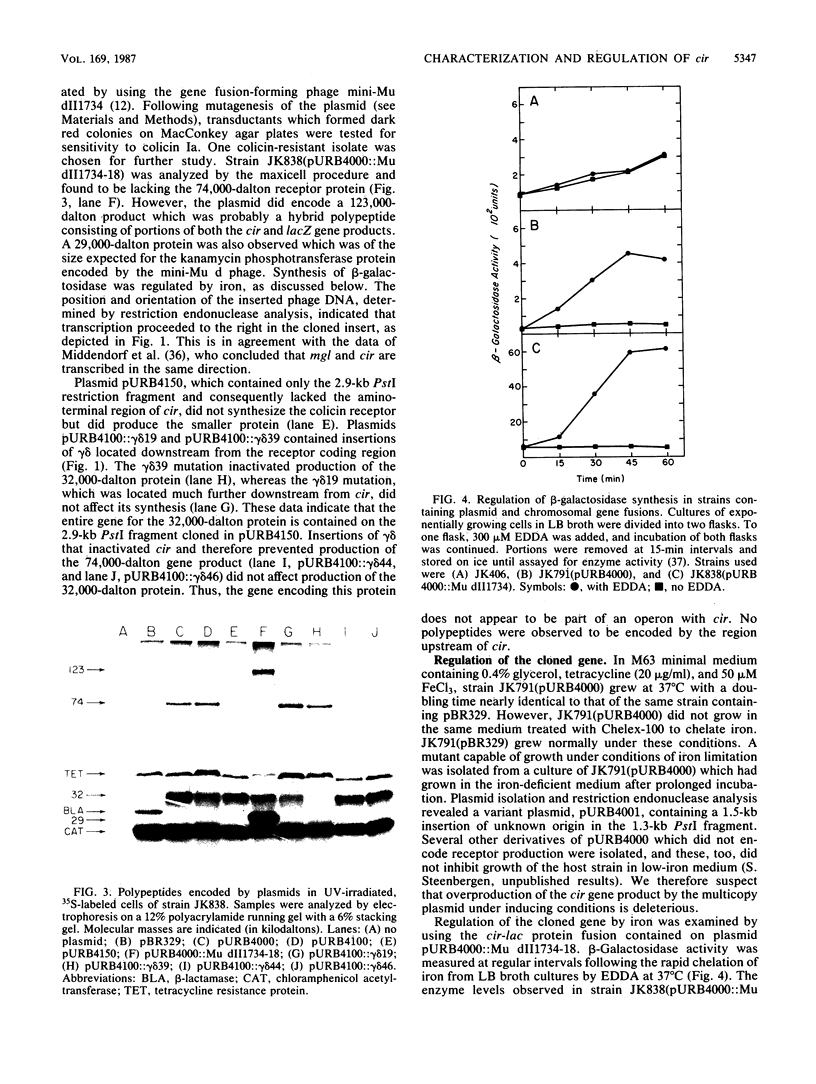
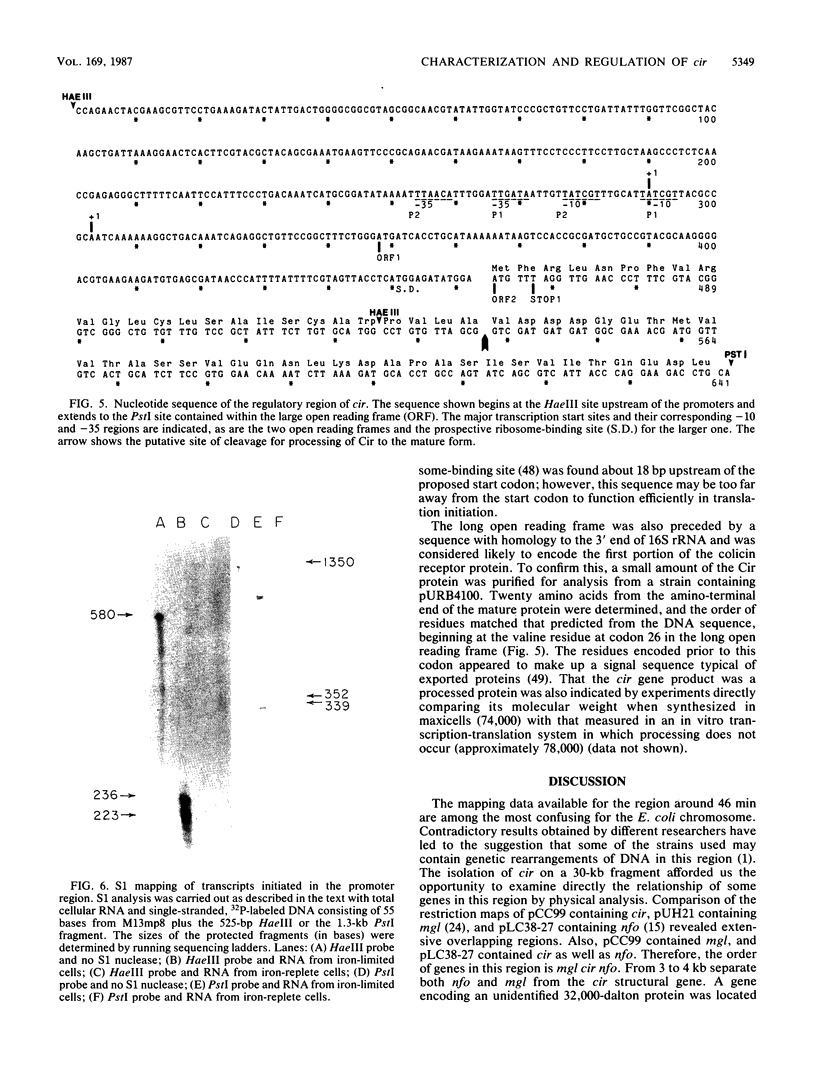
The cir gene, which encodes the colicin I receptor protein and is regulated by both cellular iron content and growth temperature, was cloned into a multicopy-number plasmid. Physical mapping and complementation analysis established the position of cir between mgl and nfo on the Escherichia coli chromosome. A gene encoding a 32,000-dalton polypeptide was located downstream of and adjacent to cir, but did not appear to be part of the same transcriptional unit. A 525-base-pair fragment from the 5' end of the 1.8-kilobase-pair receptor-coding region directed iron-regulated transcription and translation of a hybrid cir-lacZ gene. Two overlapping promoters were identified by determination of the transcriptional start sites and by sequence analysis. A small open reading frame (120 nucleotides) of unknown significance preceded the receptor-coding sequence. Examination of the amino acid sequence of the receptor purified from the outer membrane revealed that the gene product was processed by removal of a signal peptide and that the mature form had an amino acid sequence near its amino terminus which closely resembled that of several other TonB-dependent proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987 Aug 25;26(17):5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Mapping of a mutation affecting regulation of iron uptake systems in Escherichia coli K-12. J Bacteriol. 1985 Jan;161(1):450–453. doi: 10.1128/jb.161.1.450-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindereif A., Neilands J. B. Promoter mapping and transcriptional regulation of the iron assimilation system of plasmid ColV-K30 in Escherichia coli K-12. J Bacteriol. 1985 Jun;162(3):1039–1046. doi: 10.1128/jb.162.3.1039-1046.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W., Bantlow C., Benner D., Roller E. cir, a gene conferring resistance to colicin I maps between mgl and fpk on the Escherichia coli chromosome. Mol Gen Genet. 1983;191(3):401–406. doi: 10.1007/BF00425754. [DOI] [PubMed] [Google Scholar]
- Bowles L. K., Miguel A. G., Konisky J. Purification of the colicin I receptor. J Biol Chem. 1983 Jan 25;258(2):1215–1220. [PubMed] [Google Scholar]
- Braun V., Gross R., Köster W., Zimmermann L. Plasmid and chromosomal mutants in the iron(III)-aerobactin transport system of Escherichia coli. Use of streptonigrin for selection. Mol Gen Genet. 1983;192(1-2):131–139. doi: 10.1007/BF00327658. [DOI] [PubMed] [Google Scholar]
- Burke J. F. High-sensitivity S1 mapping with single-stranded [32P]DNA probes synthesized from bacteriophage M13mp templates. Gene. 1984 Oct;30(1-3):63–68. doi: 10.1016/0378-1119(84)90105-7. [DOI] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., Cameron D. R., Carmel G., Jean R., Rode H. N. Protein fusions of beta-galactosidase to the ferrichrome-iron receptor of Escherichia coli K-12. J Bacteriol. 1986 Jan;165(1):181–192. doi: 10.1128/jb.165.1.181-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. VI. Plasmid pBR329, a new derivative of pBR328 lacking the 482-base-pair inverted duplication. Gene. 1982 Jan;17(1):79–89. doi: 10.1016/0378-1119(82)90103-2. [DOI] [PubMed] [Google Scholar]
- Cunningham R. P., Saporito S. M., Spitzer S. G., Weiss B. Endonuclease IV (nfo) mutant of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1120–1127. doi: 10.1128/jb.168.3.1120-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group B. J Bacteriol. 1975 Jul;123(1):96–101. doi: 10.1128/jb.123.1.96-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Fecker L., Braun V. Cloning and expression of the fhu genes involved in iron(III)-hydroxamate uptake by Escherichia coli. J Bacteriol. 1983 Dec;156(3):1301–1314. doi: 10.1128/jb.156.3.1301-1314.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick K. K., Quackenbush R. L., Konisky J. Cloning of immunity and structural genes for colicin V. J Bacteriol. 1981 Nov;148(2):498–507. doi: 10.1128/jb.148.2.498-507.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S. The gamma delta sequence of F is an insertion sequence. J Mol Biol. 1978 Dec 15;126(3):347–365. doi: 10.1016/0022-2836(78)90045-1. [DOI] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Harayama S., Bollinger J., Iino T., Hazelbauer G. L. Characterization of the mgl operon of Escherichia coli by transposon mutagenesis and molecular cloning. J Bacteriol. 1983 Jan;153(1):408–415. doi: 10.1128/jb.153.1.408-415.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K., Kadner R. J. Nucleotide sequence of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1985 Mar;161(3):904–908. doi: 10.1128/jb.161.3.904-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Klebba P. E., McIntosh M. A., Neilands J. B. Kinetics of biosynthesis of iron-regulated membrane proteins in Escherichia coli. J Bacteriol. 1982 Mar;149(3):880–888. doi: 10.1128/jb.149.3.880-888.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krone W. J., Oudega B., Stegehuis F., de Graaf F. K. Cloning and expression of the cloacin DF13/aerobactin receptor of Escherichia coli (ColV-K30). J Bacteriol. 1983 Feb;153(2):716–721. doi: 10.1128/jb.153.2.716-721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köck J., Olschläger T., Kamp R. M., Braun V. Primary structure of colicin M, an inhibitor of murein biosynthesis. J Bacteriol. 1987 Jul;169(7):3358–3361. doi: 10.1128/jb.169.7.3358-3361.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Peters R., Bernheimer H., Berendsen W. Influence of cultural conditions and mutations on the composition of the outer membrane proteins of Escherichia coli. Mol Gen Genet. 1976 Sep 23;147(3):251–262. doi: 10.1007/BF00582876. [DOI] [PubMed] [Google Scholar]
- Lundrigan M. D., Kadner R. J. Nucleotide sequence of the gene for the ferrienterochelin receptor FepA in Escherichia coli. Homology among outer membrane receptors that interact with TonB. J Biol Chem. 1986 Aug 15;261(23):10797–10801. [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Middendorf A., Schweizer H., Vreemann J., Boos W. Mapping of markers in the gyrA-his region of Escherichia coli. Mol Gen Genet. 1984;197(1):175–181. doi: 10.1007/BF00327939. [DOI] [PubMed] [Google Scholar]
- Murphy J. R., Michel J. L., Teng M. Evidence that the regulation of diphtheria toxin production is directed at the level of transcription. J Bacteriol. 1978 Aug;135(2):511–516. doi: 10.1128/jb.135.2.511-516.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Pierce J. R., Earhart C. F. Escherichia coli K-12 envelope proteins specifically required for ferrienterobactin uptake. J Bacteriol. 1986 Jun;166(3):930–936. doi: 10.1128/jb.166.3.930-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. R., Mottur G. P., Bradbeer C. Transport of vitamin B12 in Escherichia coli. Some observations on the roles of the gene products of BtuC and TonB. J Biol Chem. 1980 May 10;255(9):4313–4319. [PubMed] [Google Scholar]
- Reznikoff W. S., Siegele D. A., Cowing D. W., Gross C. A. The regulation of transcription initiation in bacteria. Annu Rev Genet. 1985;19:355–387. doi: 10.1146/annurev.ge.19.120185.002035. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm E., Mende J., Braun V., Kamp R. M. Nucleotide sequence of the colicin B activity gene cba: consensus pentapeptide among TonB-dependent colicins and receptors. J Bacteriol. 1987 Jul;169(7):3350–3357. doi: 10.1128/jb.169.7.3350-3357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäffer S., Hantke K., Braun V. Nucleotide sequence of the iron regulatory gene fur. Mol Gen Genet. 1985;200(1):110–113. doi: 10.1007/BF00383321. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Burgi E. Properties of a membrane-attached form of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):91–105. doi: 10.1016/0022-2836(74)90576-2. [DOI] [PubMed] [Google Scholar]
- Worsham P. L., Konisky J. Effect of growth temperature on the acquisition of iron by Salmonella typhimurium and Escherichia coli. J Bacteriol. 1984 Apr;158(1):163–168. doi: 10.1128/jb.158.1.163-168.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsham P. L., Konisky J. Locus affecting regulation of the colicin I receptor by iron. J Bacteriol. 1985 Jan;161(1):428–431. doi: 10.1128/jb.161.1.428-431.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsham P. L., Konisky J. Use of cir-lac operon fusions to study transcriptional regulation of the colicin Ia receptor in Escherichia coli K-12. J Bacteriol. 1981 Jan;145(1):647–650. doi: 10.1128/jb.145.1.647-650.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Kaplan S. Effects of light, oxygen, and substrates on steady-state levels of mRNA coding for ribulose-1,5-bisphosphate carboxylase and light-harvesting and reaction center polypeptides in Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Jun;162(3):925–932. doi: 10.1128/jb.162.3.925-932.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann L., Hantke K., Braun V. Exogenous induction of the iron dicitrate transport system of Escherichia coli K-12. J Bacteriol. 1984 Jul;159(1):271–277. doi: 10.1128/jb.159.1.271-277.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Veaux L. C., Clevenson D. S., Bradbeer C., Kadner R. J. Identification of the btuCED polypeptides and evidence for their role in vitamin B12 transport in Escherichia coli. J Bacteriol. 1986 Sep;167(3):920–927. doi: 10.1128/jb.167.3.920-927.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]