Abstract
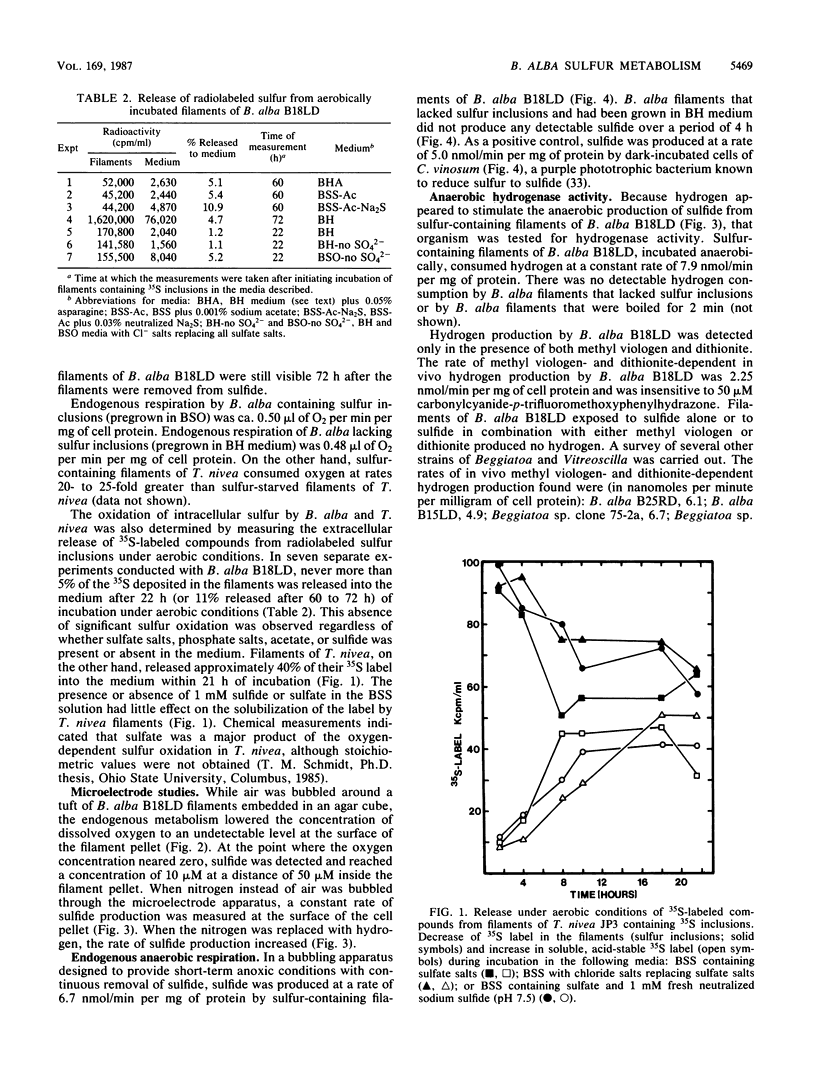
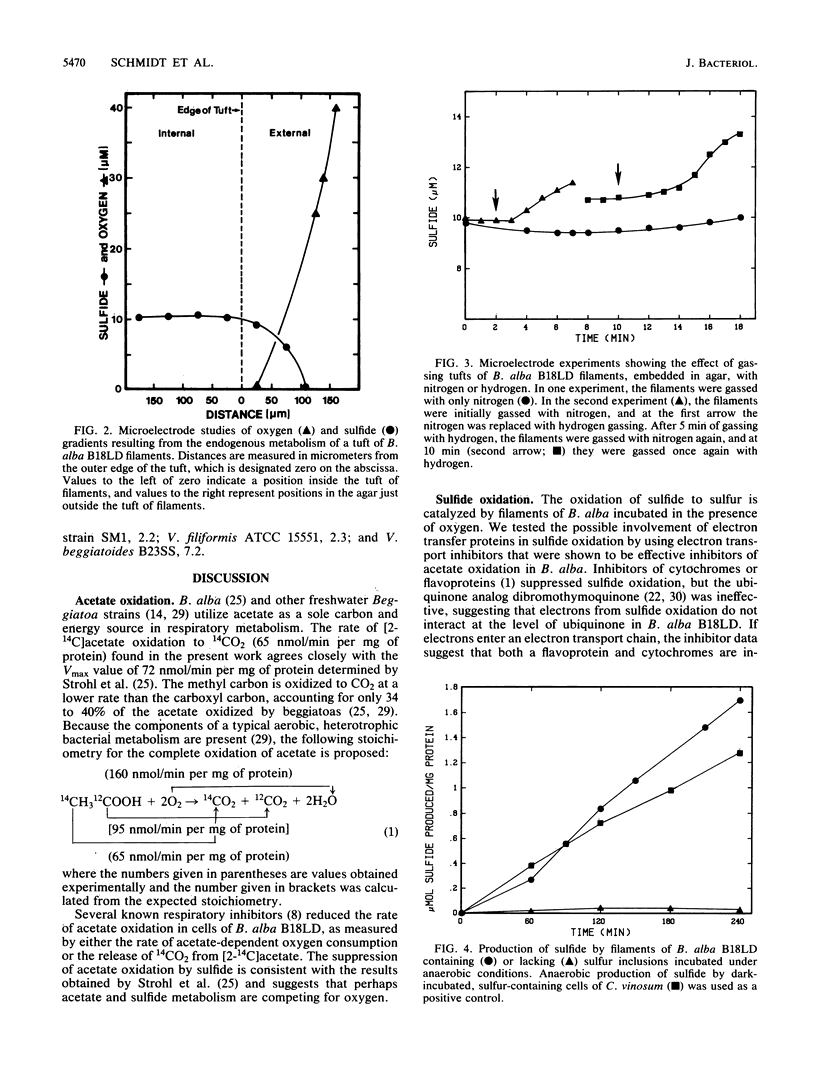
The metabolism of sulfide, sulfur, and acetate by Beggiatoa alba was investigated under oxic and anoxic conditions. B. alba oxidized acetate to carbon dioxide with the stoichiometric reduction of oxygen to water. In vivo acetate oxidation was suppressed by sulfide and by several classic respiratory inhibitors, including dibromothymoquinone, an inhibitor specific for ubiquinones. B. alba also carried out an oxygen-dependent conversion of sulfide to sulfur, a reaction that was inhibited by several electron transport inhibitors but not by dibromothymoquinone, indicating that the electrons released from sulfide oxidation were shuttled to oxygen without the involvement of ubiquinones. Intracellular sulfur stored by B. alba was not oxidized to sulfate or converted to an external soluble form under aerobic conditions. On the other hand, sulfur stored by filaments of Thiothrix nivea was oxidized to extracellular soluble oxidation products, including sulfate. Sulfur stored by filaments of B. alba, however, was reduced to sulfide under short-term anoxic conditions. This anaerobic reduction of sulfur was linked to the endogenous oxidation of stored carbon and to hydrogen oxidation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biebl H., Pfennig Growth of sulfate-reducing bacteria with sulfur as electron acceptor. Arch Microbiol. 1977 Feb 4;112(1):115–117. doi: 10.1007/BF00446664. [DOI] [PubMed] [Google Scholar]
- Fauque G., Herve D., Le Gall J. Structure-function relationship in hemoproteins: the role of cytochrome c3 in the reduction of colloidal sulfur by sulfate-reducing bacteria. Arch Microbiol. 1979 Jun;121(3):261–264. doi: 10.1007/BF00425065. [DOI] [PubMed] [Google Scholar]
- Güde H., Strohl W. R., Larkin J. M. Mixotrophic and heterotrophic growth of Beggiatoa alba in continuous culture. Arch Microbiol. 1981 Jul;129(5):357–360. doi: 10.1007/BF00406462. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nelson D. C., Castenholz R. W. Organic nutrition of Beggiatoa sp. J Bacteriol. 1981 Jul;147(1):236–247. doi: 10.1128/jb.147.1.236-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. C., Castenholz R. W. Use of reduced sulfur compounds by Beggiatoa sp. J Bacteriol. 1981 Jul;147(1):140–154. doi: 10.1128/jb.147.1.140-154.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. C., Jørgensen B. B., Revsbech N. P. Growth Pattern and Yield of a Chemoautotrophic Beggiatoa sp. in Oxygen-Sulfide Microgradients. Appl Environ Microbiol. 1986 Aug;52(2):225–233. doi: 10.1128/aem.52.2.225-233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. C., Revsbech N. P., Jørgensen B. B. Microoxic-Anoxic Niche of Beggiatoa spp.: Microelectrode Survey of Marine and Freshwater Strains. Appl Environ Microbiol. 1986 Jul;52(1):161–168. doi: 10.1128/aem.52.1.161-168.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A., Padan E. Induction of anaerobic, photoautotrophic growth in the cyanobacterium Oscillatoria limnetica. J Bacteriol. 1978 Feb;133(2):558–563. doi: 10.1128/jb.133.2.558-563.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Paschinger H., Paschinger J., Gaffron H. Photochemical disproportionation of sulfur into sulfide and sulfate by Chlorobium limicola forma thiosulfatophilum. Arch Mikrobiol. 1974 Mar 28;96(4):341–351. [PubMed] [Google Scholar]
- Poole R. K., Haddock B. A. Dibromothymoquinone : an inhibitor of aerobic electron transport at the level of ubiquinone in Escherichia coli. FEBS Lett. 1975 Mar 15;52(1):13–16. doi: 10.1016/0014-5793(75)80626-0. [DOI] [PubMed] [Google Scholar]
- Strohl W. R., Cannon G. C., Shively J. M., Güde H., Hook L. A., Lane C. M., Larkin J. M. Heterotrophic carbon metabolism by Beggiatoa alba. J Bacteriol. 1981 Nov;148(2):572–583. doi: 10.1128/jb.148.2.572-583.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl W. R., Larkin J. M. Enumeration, isolation, and characterization of beggiatoa from freshwater sediments. Appl Environ Microbiol. 1978 Nov;36(5):755–770. doi: 10.1128/aem.36.5.755-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun I. L., Crane F. L. Coordinated, coenzyme Q reversible, 2,5-dibromothymoquionine inhibition of electron transport and ATPase in Escherichia coli. Biochem Biophys Res Commun. 1976 Jan 12;68(1):190–196. doi: 10.1016/0006-291x(76)90028-0. [DOI] [PubMed] [Google Scholar]
- van Gemerden H. On the ATP generation by Chromatium in darkness. Arch Mikrobiol. 1968;64(2):118–124. doi: 10.1007/BF00406970. [DOI] [PubMed] [Google Scholar]


