Abstract
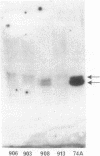
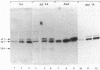
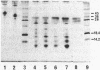
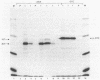
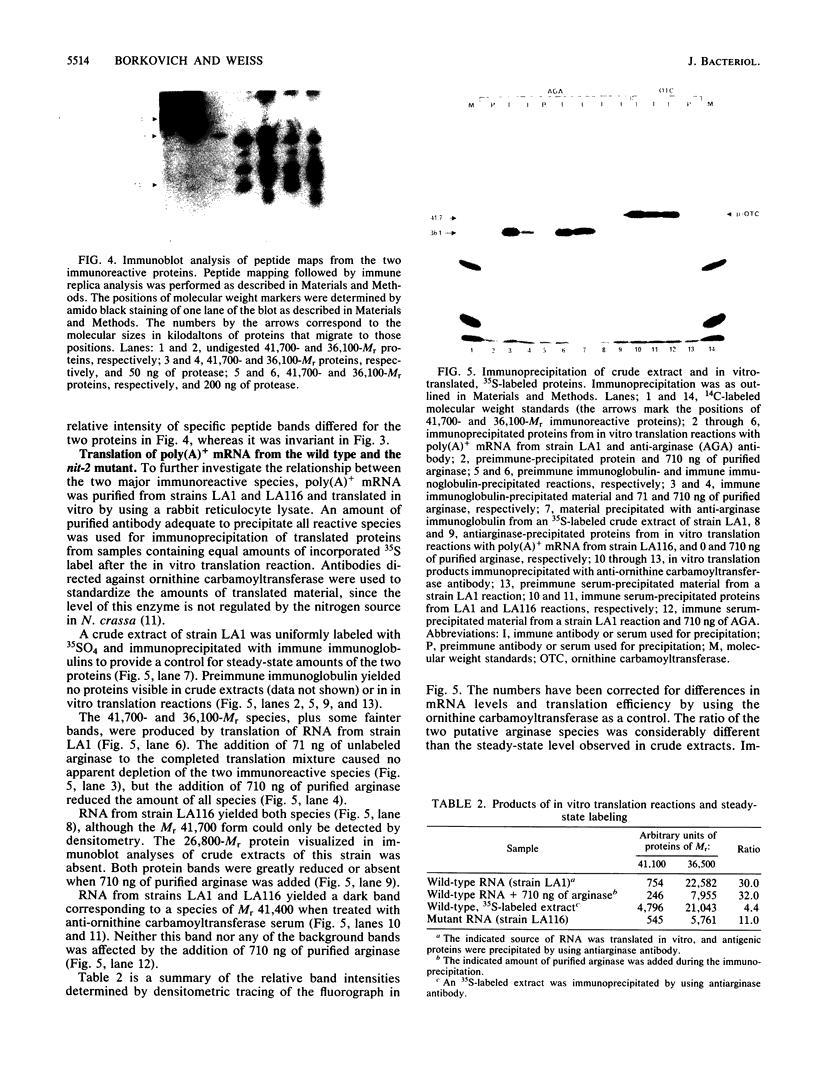
Two major immunoreactive proteins of Mr 41,700 and 36,100 have been detected in crude mycelial extracts with polyclonal antibodies raised against arginase purified from Neurospora crassa. The latter corresponded to the protein used to obtain the antibodies. Both polypeptides were either missing or present in very low amounts in mutant strains having little or no detectable arginase activity. The relative proportion of the two species was altered in strains containing the nitrogen catabolite regulatory mutation nit-2. Peptide mapping indicated that the two species are very closely related, but several of the peptides which appeared to be identical by staining reacted differently with the antibodies. Both species were produced by in vitro translation of poly(A)+ mRNA, although the larger species was produced to a much smaller extent than was expected from its abundance in vivo. The results suggest the existence of multiple forms of arginase in N. crassa which differ in their response to nitrogen catabolite regulation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Anderson P. J. Purification and quantitation of glutaraldehyde and its effect on several enzyme activities in skeletal muscle. J Histochem Cytochem. 1967 Aug;15(11):652–661. doi: 10.1177/15.11.652. [DOI] [PubMed] [Google Scholar]
- Barańczyk-Kuźma A., Porembska Z., Mochnacka I. Oligomeric structure of A1 arginase from rat liver and A4 from kidney. Difference in charge of subunits. Acta Biochim Pol. 1976;23(2-3):151–163. [PubMed] [Google Scholar]
- Berüter J., Colombo J. P., Bachmann C. Purification and properties of arginase from human liver and erythrocytes. Biochem J. 1978 Nov 1;175(2):449–454. doi: 10.1042/bj1750449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovich K. A., Weiss R. L. Purification and characterization of arginase from Neurospora crassa. J Biol Chem. 1987 May 25;262(15):7081–7086. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chin D. J., Luskey K. L., Faust J. R., MacDonald R. J., Brown M. S., Goldstein J. L. Molecular cloning of 3-hydroxy-3-methylglutaryl coenzyme a reductase and evidence for regulation of its mRNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7704–7708. doi: 10.1073/pnas.79.24.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cybis J., Davis R. H. Organization and control in the arginine biosynthetic pathway of Neurospora. J Bacteriol. 1975 Jul;123(1):196–202. doi: 10.1128/jb.123.1.196-202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Mora J. Mutants of Neurospora crassa deficient in ornithine-delta-transmainase. J Bacteriol. 1968 Aug;96(2):383–388. doi: 10.1128/jb.96.2.383-388.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn-Coleman N. S., Garrett R. H. The role fo glutamine synthetase and glutamine metabolism in nitrogen metabolite repression, a regulatory phenomenon in the lower eukaryote Neurospora crassa. Mol Gen Genet. 1980;179(1):25–32. doi: 10.1007/BF00268442. [DOI] [PubMed] [Google Scholar]
- Facklam T. J., Marzluf G. A. Nitrogen regulation of amino acid catabolism in Neurospora crassa. Biochem Genet. 1978 Apr;16(3-4):343–354. doi: 10.1007/BF00484090. [DOI] [PubMed] [Google Scholar]
- Herzfeld A., Raper S. M. The heterogeneity of arginases in rat tissues. Biochem J. 1976 Feb 1;153(2):469–478. doi: 10.1042/bj1530469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch-Kolb H., Greenberg D. M. Molecular characteristics of rat liver arginase. J Biol Chem. 1968 Dec 10;243(23):6123–6129. [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Analysis of cotranslational proteolytic processing of nascent chains using two-dimensional gel electrophoresis. Methods Enzymol. 1983;97:77–85. doi: 10.1016/0076-6879(83)97121-5. [DOI] [PubMed] [Google Scholar]
- Kaysen G. A., Strecker H. J. Purification and properties of arginase of rat kidney. Biochem J. 1973 Aug;133(4):779–788. doi: 10.1042/bj1330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Legerton T. L., Weiss R. L. Mobilization of sequestered metabolities into degradative reactions by nutritional stress in Neurospora. J Bacteriol. 1979 Jun;138(3):909–914. doi: 10.1128/jb.138.3.909-914.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenguy F., Dubois E. Participation of transcriptional and post-transcriptional regulatory mechanisms in the control of arginine metabolism in yeast. Mol Gen Genet. 1983;189(1):148–156. doi: 10.1007/BF00326068. [DOI] [PubMed] [Google Scholar]
- Ness S. A., Weiss R. L. Carbamoyl-phosphate synthetases from Neurospora crassa. Immunological relatedness of the enzymes from Neurospora, bacteria, yeast, and mammals. J Biol Chem. 1985 Nov 15;260(26):14355–14362. [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Methylmercury hydroxide enhancement of translation and transcription of ovalbumin and conalbumin mRNA's. J Biol Chem. 1979 Aug 25;254(16):7636–7642. [PubMed] [Google Scholar]
- Perlman D., Raney P., Halvorson H. O. Cytoplasmic and secreted Saccharomyces cerevisiae invertase mRNAs encoded by one gene can be differentially or coordinately regulated. Mol Cell Biol. 1984 Sep;4(9):1682–1688. doi: 10.1128/mcb.4.9.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert W. R., Marzluf G. A. Genetic and metabolic control of the purine catabolic enzymes of Neurospora crasse. Mol Gen Genet. 1975 Aug 5;139(1):39–55. doi: 10.1007/BF00267994. [DOI] [PubMed] [Google Scholar]
- Reitzer L. J., Magasanik B. Expression of glnA in Escherichia coli is regulated at tandem promoters. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1979–1983. doi: 10.1073/pnas.82.7.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypek-Osiecka I., Porembska Z. Hybridization of subunits of rat liver arginase A1 and rat kidney arginase A4. Acta Biochim Pol. 1983;30(1):93–97. [PubMed] [Google Scholar]
- Skrzypek-Osiecka I., Robin Y., Porembska Z. Purification of rat kidney arginases A1 and A4 and their subcellular distribution. Acta Biochim Pol. 1983;30(1):83–92. [PubMed] [Google Scholar]
- Spector E. B., Rice S. C., Moedjono S., Bernard B., Cederbaum S. D. Biochemical properties of arginase in human adult and fetal tissues. Biochem Med. 1982 Oct;28(2):165–175. doi: 10.1016/0006-2944(82)90067-9. [DOI] [PubMed] [Google Scholar]
- Tomsett A. B., Dunn-Coleman N. S., Garrett R. H. The regulation of nitrate assimilation in Neurospora crassa: the isolation and genetic analysis of nmr-1 mutants. Mol Gen Genet. 1981;182(2):229–233. doi: 10.1007/BF00269662. [DOI] [PubMed] [Google Scholar]
- Vaca G., Mora J. Nitrogen regulation of arginase in Neurospora crassa. J Bacteriol. 1977 Sep;131(3):719–725. doi: 10.1128/jb.131.3.719-725.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiss R. L. Compartmentation and control of arginine metabolism in Neurospora. J Bacteriol. 1976 Jun;126(3):1173–1179. doi: 10.1128/jb.126.3.1173-1179.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]