Abstract
We are interested in the signaling between axons and glia that leads to myelination and maintenance of the myelin internode, and we have focused on the role of neuregulins and their receptors. Neuregulins are a family of ligands that includes heregulin, neu differentiation factor, glial growth factor, and the acetylcholine receptor–inducing activity. Three signal transducing transmembrane receptors for neuregulins, which bear significant homology to the EGF receptor, are currently known: HER2 (erbB2), HER3 (erbB3), and HER4 (erbB4). We have found that oligodendrocite–type II astrocyte (O2A) progenitor cells and mature oligodendrocytes express HER2 and HER4 but no HER3. Schwann cells express HER2 and HER3 but little HER4. In O2A progenitor cells and oligodendrocytes, recombinant neuregulin induces the rapid tyrosine phosphorylation of only HER4. HER2 is not phosphorylated in cells of the oligodendrocyte lineage, but a physical interaction between HER2 and HER4 was detected in coimmunoprecipitation experiments. In Schwann cells, neuregulin induces the phosphorylation of both HER2 and HER3. Coimmunoprecipitation experiments indicate that receptor activation in Schwann cells results in the formation of HER2:HER3 heterodimers. Neuregulin localized immunocytochemically was present on neurites of cultured dorsal root ganglion neurons, and it was released into the medium in a form that promoted receptor tyrosine phosphorylation. Neuregulins therefore meet important criteria expected of molecules involved in axonal-glial signaling. The use of unique neuregulin receptor combinations in oligodendrocytes and Schwann cells likely results in recruitment of different signaling pathways and thus provides a basis for different biological responses.
Based on work of Richard and Mary Bunge, Patrick Wood, and their colleagues, it was known for some time that axons contain a mitogen(s) for Schwann cells (5, 61, 62, 73). In 1978 a factor in pituitary extracts was found to induce proliferation of Schwann cells (58) and was named glial growth factor (GGF)1 (33). GGF thus became a candidate for the axonal mitogen. Amino acid sequence suggested that GGFs belonged to a family of ligands now known collectively as neuregulins (20, 39) and allowed cloning of full-length cDNAs (39). Neuregulin has been used as a generic term for the homologous molecules heregulin, neu differentiation factor, acetylcholine receptor–inducing activity, and GGF, and while the term does not encompass the numerous biological activities of this family of molecules outside the nervous system, we will use it here for the sake of simplicity (15, 26, 39, 70). Recent experiments suggest that neuregulins are responsible for part or all of the mitogenic effect of axons on Schwann cells. Using the 2C4 antibody, which is an antagonist for one of the neuregulin receptors, Bunge and co-workers were able to block the mitogenic effect of dorsal root ganglion (DRG) neurons on Schwann cells (34, 46). In related experiments, Jessen and colleagues found that neuregulins induced survival of Schwann cell precursors (11). Conditioned medium from DRG neurons possessed a similar survival activity that was inhibited by a soluble form of a neuregulin receptor that binds ligand and blocks its ability to interact with native receptor (11).
The multiple isoforms of neuregulin originate from a single gene (human chromosome 8p12-p21), probably by use of multiple promoters and alternative mRNA splicing (51). Most neuregulin isoforms encode a transmembrane protein with a signal peptide–containing amino-terminal domain, an immunoglobulin domain, an adjacent spacer domain with N- and O-linked glycosylation sites, an EGFlike domain with six conserved cysteines, a juxtamembrane domain, a transmembrane domain, and a highly conserved cytoplasmic domain (4, 7, 52). Proteolytic cleavage of this integral membrane form of neuregulin generates an extracellular component of ∼40–50 kD that has been referred to as mature neuregulin (35, 71). The EGF-like domain represents the minimal structure necessary for binding to receptor and also for all known biological activities (8, 26). Three disulfide bonds between cysteines 1 and 3, 2 and 4, and 5 and 6 within the EGF-like domain are required for activity and do so by bridging conserved amino acids at the NH2-terminal and COOH-terminal ends of the EGF-like domain suggested to play a role in specificity for its cognate receptors (28).
Three transmembrane receptors for neuregulin are currently known—HER2/erbB2, HER3/erbB3, and HER4/ erbB4—all of which are homologous to the epidermal growth factor receptor (EGFR), which is designated as HER1/erbB1 (9, 31, 54). In common with other receptor tyrosine kinases, ligand binding to HER receptors results in activated receptor dimers. Of these receptors, HER3 and HER4, but not HER2 or HER1, directly bind neuregulin with high affinity (8, 29, 53, 54). HER2 and HER4 have functional intrinsic tyrosine kinases, whereas HER3 has markedly reduced kinase activity (66). While HER2 lacks the ability to bind soluble ligand directly, coexpression of HER2 with HER3 significantly increases ligand affinity compared with cells expressing HER3 alone (66). Many but not all of the possible hetero- and homodimers—HER2: HER3, HER2:HER4, HER3:HER4, HER4:HER4, and EGFR:HER(2, 3 or 4)—become tyrosine phosphorylated upon ligand binding and initiate signaling cascades (56, 66, 69).
In addition to their actions on Schwann cells, neuregulins also act on oligodendrocytes, the myelin-forming cells of the central nervous system (CNS), by inducing the extension of large sheetlike processes characteristic of a premyelinating phenotype (68). In oligodendrocytes, neuregulins induce the tyrosine phosphorylation of a 180–190-kD protein. Neuregulins clearly have different effects on the myelin-forming cells of the CNS and peripheral nervous system (PNS), and we investigated if the signal transducing neuregulin receptors used by CNS and PNS glia differed. Neuregulin receptor usage will determine in part the signaling pathways activated and thus the biological response of a cell to neuregulins. In this report, we present data showing oligodendrocyte–type II astrocyte (O2A) progenitor cells and oligodendrocytes express the neuregulin receptors HER2 and HER4, while Schwann cells express HER2 and HER3. Neuregulin induces the rapid phosphorylation of HER4 in cells of the oligodendrocyte lineage and both HER2 and HER3 in Schwann cells. We determined that DRG neurons, which have both CNS and PNS myelinated axons, express multiple neuregulin isoforms and demonstrate that neuregulin derived from DRG neurons is biologically active.
Materials and Methods
Materials
The antigalactosylcerebroside monoclonal IgM, O1, was generated by Dr. M. Schachner and colleagues, and the hybridoma was obtained from Dr. Stephen Pfeiffer (University of Connecticut, Farmington, CT). Polyclonal and monoclonal antibodies for HER2, HER3, HER4, and neuregulin were obtained from Santa Cruz Laboratories (Santa Cruz, CA). Antiphosphotyrosine monoclonal antibody was G410.
Isolation of O2A Progenitor Cells
In the forebrain, oligodendrocytes arise in the subventricular zone (19) from a bipolar cell that bears the surface marker A2B5, but not O4 or O1 (17). This cell is termed an oligodendrocyte–type II astrocyte (O2A) progenitor cell because in vitro it has the potential to develop into an oligodendrocyte or an astrocyte depending on culture conditions (59). In vivo the O2A progenitor cell appears to develop predominantly along oligodendrocyte lineage. O2A progenitor cells were isolated from the forebrains of 1-d-old Sprague Dawley rats using a previously described method with slight modifications (68). After detachment of progenitors from crude mixtures by a 15-h, 180-rpm shake, cells were exposed to leucine methyl ester during three successive 15-min tissue culture plastic panning steps. This procedure reduced microglia to <0.5% of the plating mixture. Progenitors depleted of microglia were then plated onto polyornithine-coated 22-mm glass coverslips or 60-mm tissue culture dishes in 5% FCS and switched to defined medium for oligodendrocytes (DMoli) 30 min after plating (day 0). DMoli consisted of DME (GIBCO BRL, Grand Island, NY), 0.5% recrystalized BSA, 5 μg/ml insulin, 100 μM putracine, 50 μg/ml transferrin, 5 ng/ml selenium, 30 nM triiodothyronine, 20 nM progesterone, and 10 ng/ml D-biotin (Sigma Chemical Co., St. Louis, MO).
Isolation of Schwann Cells
Schwann cells were isolated from postnatal day 0–1 (P0–P1) rat sciatic nerves. Sciatic nerves were digested in 0.1% collagenase and 0.25% trypsin and triturated with a 1-ml pipet ten times and then a fire-polished pasture pipet seven times in 10% FBS. Dissociated cells from 40 sciatic nerves were resuspended in 5 ml of DME with 10% FBS and plated into a 25-cm2 flask. On the second day of culture, cells were treated with Ara-C for 24 h to reduce fibroblast contamination. On day 4, cultures were treated with GGF and forskolin (5 μM) to expand the Schwann cell population. On day 7 in vitro, cultures were trypsinized, washed, and treated with anti-Thy 1.1 followed by complement to eliminate remaining fibroblasts. Cells were plated onto polylysine-coated tissue culture dishes or cover glasses for subsequent experiments.
Isolation of DRG Neurons
DRG neurons were isolated by digesting E15 rat DRGs in trypsin (0.125% wt/vol) and collagenase (0.5% wt/vol) for 10–20 min, triturating seven times with a fire-polished pasture pipet in 10% FBS, and then allowing large undigested fragments to settle. Supernatants were collected by centrifugation and pellets resuspended in DME with N2 additives, 2% rat serum, and 50 ng/ml of NGF. Cells were plated onto polylysine- and laminin-coated dishes and pulsed with 1 μM fluorodeoxyuridine and uridine as described by (29a). Cultures usually required two to three fluorodeoxyuridine treatments to significantly reduce the numbers of Schwann cells and fibroblasts.
Immunohistochemistry
Cultures of O2A progenitor cells, oligodendrocytes, Schwann cells, or DRG neurons were fixed in fresh 4% paraformaldehyde in PBS (wt/vol) for 7 min at ambient temperature, washed three times with PBS, permeabilized with 0.125% Triton X-100 in PBS (vol/vol) for 20 min at ambient temperature, blocked with 5% nonfat dry milk in PBS (wt/vol) for 1 h, incubated with primary antibody in blocking solution overnight at 4°C, washed three times with PBS, and then incubated with Cy3-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA).
Immunoprecipitation
Cultures of O2A progenitor cells or oligodendrocytes in 60-mm tissue culture dishes were harvested on ice in lysis buffer (150 mM NaCl, 20 mM Tris, 1 mM EDTA, 1.0% NP-40, 1 mM PMSF, 100 μg/ml leupeptin, 1 mM sodium orthovanadate). Lysates were gently rotated at 4°C for 2 h and cleared of insoluble material by precipitation at 10,000 g for 5 min. Lysates were then incubated 6–12 h with 1 μg/ml of primary antibody at 4°C with gentle rotation. Antibody antigen complexes were precipitated after a 1-h incubation with protein A–conjugated Sepharose 4B at 4°C with gentle rotation. Immunoprecipitates were solubilized with loading buffer (10% SDS, Tris, β-mercaptoethanol, bromophenol blue), resolved on polyacrylamide gels (5 or 4–15% gradient as indicated), and then transferred to polyvinyl difluoride (PVDF) membranes (Millipore Corp., Bedford, MA).
Western Blotting
PVDF membranes with transferred protein were blocked with 3% nonfat dry milk in PBS (wt/vol), pH 7.4, for 1 h at ambient temperature. Blocked membranes were then incubated with primary antibody in PBS/milk for 2 h at ambient temperature, washed four times in PBS, incubated with the appropriate peroxidase-conjugated secondary antibody for 1 h at ambient temperature, and then washed four times for 15 min with PBS, and once for 5 min with 0.05% Tween-20 in PBS (vol/vol). Washed blots were incubated for 1 min with extended chemiluminescence reagents (New England Nuclear, Boston, MA) and then exposed to x-ray film for the times indicated.
Subcellular Fractionation
Subcellular fractions were prepared from 100-mm dishes of DRG neurons plated at a density of 1 × 104 cells/cm2 and that had been cultured 3 wk. Cultures were washed with PBS, scraped in hypotonic buffer (20 mM Hepes, pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1 mM PMSF), and lysed with 100 strokes in a tight-fitting Dounce homogenizer. Nuclei and unlysed cells were precipitated by centrifugation at 1,500 g for 5 min, and the pellet and supernatant were examined under a microscope to assure removal of nuclei and unlysed cells. The supernatant from the 1,500 g spin was then centrifuged at 100,000 g for 30 min, and the resulting supernatant was used as the cytosolic fraction and the pellet as the membrane fraction.
Conditioned Media
100-mm plates of DRG neurons ∼3 wk in vitro and free of Schwann cells were washed three times and switched to 5 ml of serum-free medium (DME with N2 additives and 50 ng/ml of NGF) for 4 d. Conditioned media were collected and centrifuged at 1,000 g to eliminate any precipitates or cell fragments. Cleared media was then applied to an Amicon (Beverly, MA) Centriprep concentrator with a 10-kD exclusion and used as described by the manufacturer. The 5× retentate was used in an assay of p185 tyrosine phosphorylation in L6 muscle cells as previously described (15).
Results
Oligodendrocytes Express Neuregulin Receptors HER2 and HER4
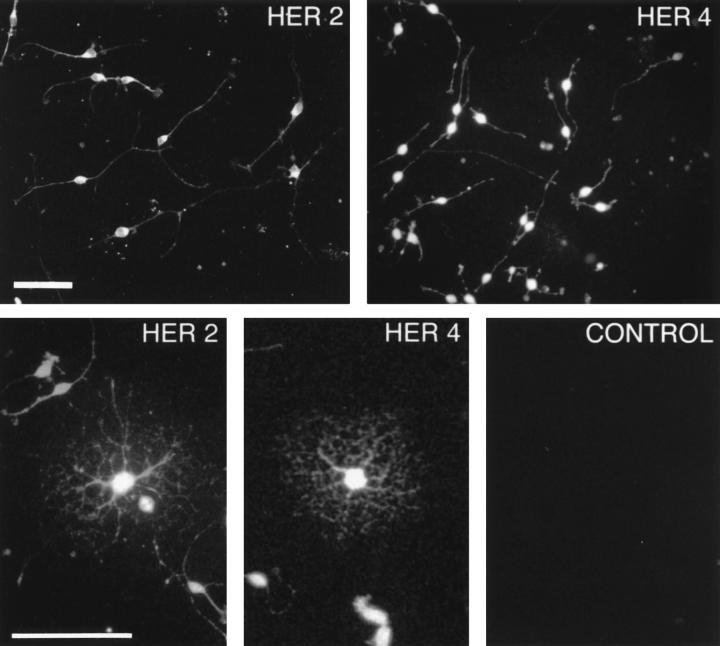
Neuregulin receptor expression by O2A progenitor cells and oligodendrocytes was evaluated by immunohistochemistry and Western blotting. O2A progenitor cells isolated from P0 rat forebrain were grown in serum-free, chemically defined medium (see Materials and Methods) in the presence of PDGF-AA and basic FGF to maintain cells as a proliferating progenitor cell population (18, 41, 72), or in the absence of these mitogens and in the presence of insulin to allow for differentiation into oligodendrocytes. Both O2A progenitor cells and oligodendrocytes stained with antibodies directed against HER2 and HER4 (Fig. 1) but not HER3 (not shown). The affinity of the HER3 antibody was adequate for immunohistochemistry as demonstrated with Schwann cell staining (see Fig. 4). Specificity of the HER2 and HER4 antibodies was shown by blocking with an excess of peptide from which the antibodies were raised (Fig. 1). 100% of O2A progenitor cells and oligodendrocytes stain for HER2 or HER4 in single labeling studies, and thus we infer that individual O2A progenitor cells and oligodendrocytes possess both HER2 and HER4. The staining pattern in O2A progenitor cells was similar but not identical for HER2 and HER4. HER2 immunoreactivity was present throughout the entire length of the bipolar processes in O2A progenitor cells, while HER4 immunoreactivity was absent in the distal-most ends of processes.
Figure 1.
HER2 and HER4 are present on cells in the oligodendrocyte lineage. O2A progenitor cells (upper) or oligodendrocytes (lower) were fixed in fresh 4% paraformaldehyde and then permeabilized for 20 min in 0.125% Triton X100. These cultures were stained with polyclonal antibodies that recognize intracellular epitopes of the neuregulin receptors and visualized using Cy3-conjugated secondary antibodies. Virtually all cells were intensely labeled. Control represents a culture of O2A progenitor cells incubated with anti-HER4 in the presence of 100-fold excess of the peptide to which the antibody was raised and similar results were obtained with HER2. Bars, 50 μm.
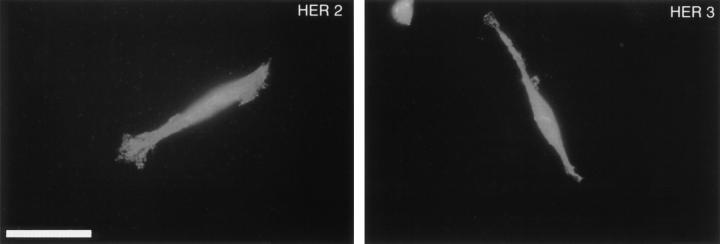
Figure 4.
HER2 and HER3 are present on Schwann cells. Schwann cells were cultured as described, fixed in 4% paraformaldehyde, permeabilized with 0.125% Triton X-100, and stained with polyclonal antisera directed against HER2 or HER3 followed by Cy3-conjugated secondary antibodies. Virtually all Schwann cells stain for both HER2 and HER3. Bar, 50 μm.
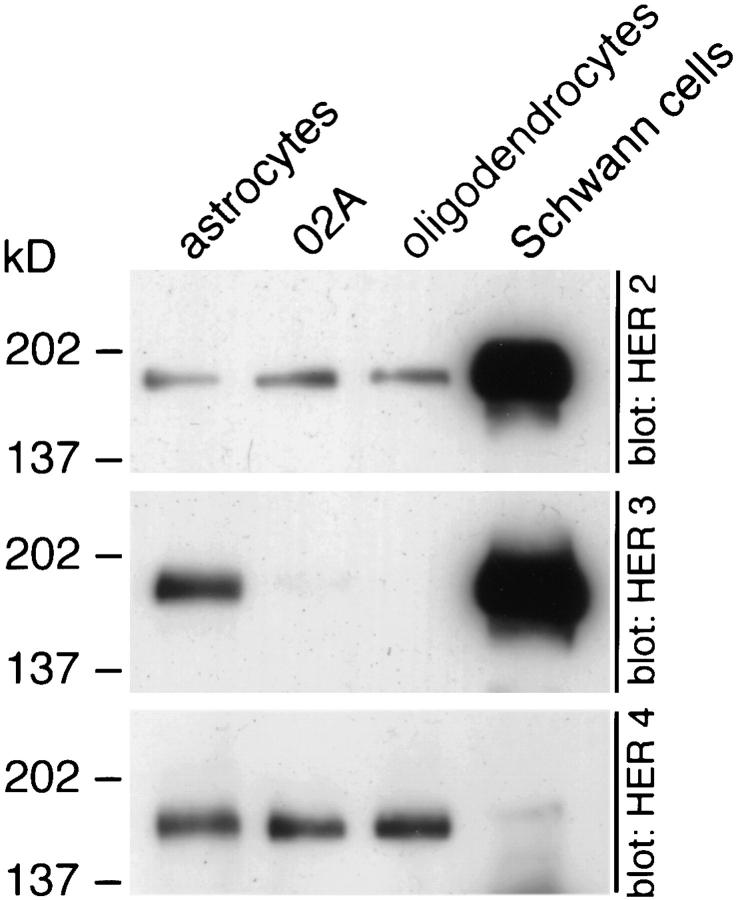
Western blot analysis confirmed the presence of significant levels of HER2 and HER4 and the absence of HER3 in total lysates of cultured O2A progenitor cells and oligodendrocytes (Fig. 2). In contrast to cells in the oligodendrocyte lineage, astrocytes express all three neuregulin receptors (Fig. 2). Unlike cells in the oligodendrocyte lineage, Schwann cells express large amounts of HER3 (Fig. 2). Schwann cells also express quantitatively more HER2 than do cells in the oligodendrocyte lineage. Schwann cells express only trace amounts of HER4 (Fig. 2). Similar experiments in both mouse and rat have shown expression of HER2 and HER3 by Schwann cells (24, 34, 45, 46).
Figure 2.
Distinct neuregulin receptor expression by CNS and PNS glial cells. Primary cultures of astrocytes, O2A progenitor cells, oligodendrocytes, and Schwann cells were prepared as described. Cell lysates containing 10 μg of protein were resolved on 4–15% gradient polyacrylamide gels and probed with HER2, HER3, or HER4 antibodies. Cells in the oligodendrocyte lineage express HER2 and HER4, Schwann cells express HER2 and HER3, and astrocytes express HER2, HER3, and HER4.
Neuregulin Induces the Tyrosine Phosphorylation of HER4 but Not HER2 in O2A Progenitor Cells and Oligodendrocytes
Cells of the oligodendrocyte lineage express HER2 and HER4, and therefore HER2:HER2, HER4:HER4, and HER2:HER4 homo- and heterodimers are possible signal transducing receptor units. In transfected cell lines, data suggest that HER2:HER2 homodimers are not functional signal transducing units because they lack the ability to bind neuregulin. To investigate receptor usage in primary glial cells, we examined O2A progenitor cells and oligodendrocytes for the receptor tyrosine phosphorylation events after treatment with neuregulin. In the upper panel of Fig. 3 A, the tyrosine phosphorylation of an ∼180-kD protein is evident in HER4 and HER2 immunoprecipitates from both neuregulin-treated O2A progenitor cells and oligodendrocytes. HER2 and HER4 may be resolved by SDSPAGE with approximate molecular masses of 185 and 180 kD, respectively, and based on relative mobility of the two receptors, it appeared that the tyrosine-phosphorylated protein was HER4 rather than HER2. To definitively assign the tyrosine-phosphorylated protein to either HER2 or HER4, we stripped the membranes and reprobed them for either HER2 (Fig. 3 A, middle) or HER4 (Fig. 3 A, lower). Based on alignment of the resultant autoradiograms, it was clear that the tyrosine-phosphorylated band comigrated with HER4 and not HER2. Significant amounts of HER4 were present in HER2 immunoprecipitates in both ligand-treated and control cultures, but little HER2 was detected in HER4 immunoprecipitates. This suggested to us that HER2:HER4 heterodimers may exist in the presence and absence of ligand. Furthermore, the fact that HER4 is present in HER2 immunoprecipitates but not the converse suggests that HER4 is in excess of HER2 and that HER4 homodimers actually form. The definitive demonstration of HER4 homodimers will require chemical cross-linking studies.
Figure 3.
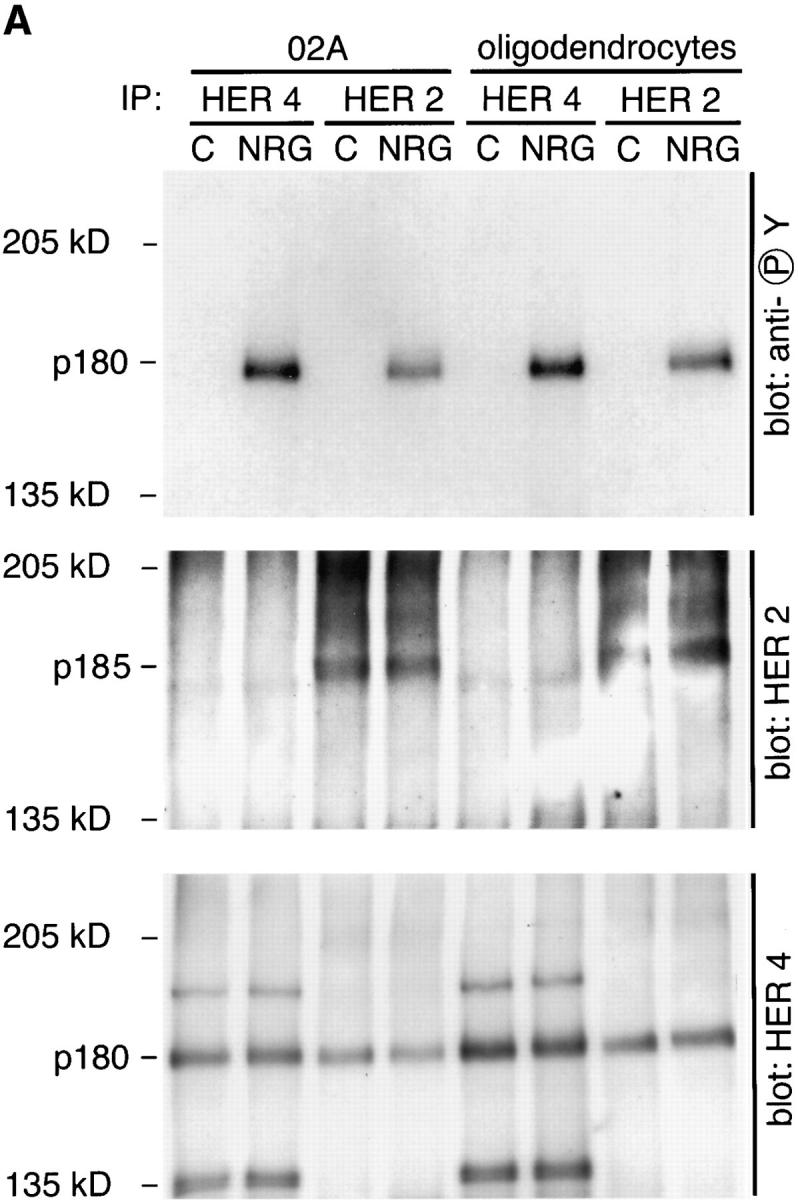
Neuregulin-induced tyrosine phosphorylation of HER4 in O2A progenitor cells and oligodendrocytes. (A) Cultures were treated with 100 pM neuregulin (EGF-like β1 domain of neu differentiation factor) or control buffer (0.1% recrystalized BSA in PBS) for 5 min, harvested, and HER4 or HER2 immunoprecipitated as described. All three panels represent autoradiographs of the same membrane probed sequentially with three different primary antibodies: monoclonal antiphosphotyrosine (upper), polyclonal anti-HER2 (middle), and anti-HER4 (lower). Before reprobing the membrane, primary and secondary antibody complexes were stripped as described in Materials and Methods. In both O2A progenitor cells and oligodendrocytes, neuregulin induces the phosphorylation of a band at ∼180-kD that comigrates with HER4 but not HER2. HER4 is seen in the HER2 immunoprecipitates regardless of neuregulin treatment, indicating an association of the two receptors in the absence of ligand. The bands at ∼190 and 135 kD recognized by HER4 antibodies in HER4 immunoprecipitates (lower) are of unknown significance but may represent unprocessed and truncated forms of the receptor. (B) Time course of HER4 phosphorylation. Cultures of O2A progenitors were treated for the times indicated and HER4 was immunoprecipitated. Immunoprecipitates were treated as above using monoclonal antiphosphotyrosine as the primary antibody, and the autoradiograph is shown.
HER4 is phosphorylated rapidly in cells of the oligodendrocyte lineage. In the experiment shown in Fig. 3 B, O2A progenitor cells were treated for the times indicated with neuregulin, and HER4 was immunoprecipitated. Tyrosine phosphorylation of HER4 in O2A progenitor cells was detectable 60 s after exposure to neuregulin and persisted for >1 h (Fig. 3 B).
Schwann Cells Express Neuregulin Receptors HER2 and HER3
In contrast to cells of the oligodendrocyte lineage, neuregulins induce a mitogenic response in Schwann cells (20, 39, 46, 68). We sought to determine if the different biological responses of CNS and PNS myelin-forming cells to neuregulins could be accounted for by different neuregulin receptors used by each cell type. In contrast to oligodendrocytes and O2A progenitor cells, cultured Schwann cells labeled intensely for HER3 as well as HER2 (Fig. 4). The labeling pattern for both receptors was the same, staining processes and the cell body equivalently.
Neuregulin Induces the Tyrosine Phosphorylation of HER2 and HER3 in Schwann Cells
We examined total lysates of Schwann cells and found that neuregulin induced the rapid tyrosine phosphorylation of a 170–190-kD protein detected 1 min after treatment, peaking in intensity at 30 min (Fig. 5). At the time of peak tyrosine phosphorylation, a mobility shift in the HER2 band was observed (Fig. 5). This suggests that the change in electrophoretic properties results from the increased phosphorylation, as has been noted with other proteins (27). After more prolonged treatment, the HER2 band migrated to its pretreatment mobility. Furthermore, the amount of HER2 in these total lysates had apparently decreased at later time points, perhaps reflecting ligand-dependent degradation (Fig. 5). Neuregulin also stimulates a mobility shift in the HER3 immunoreactive band over the same time course (Fig. 5). Unlike HER2 however, the intensity and mobility of the HER3 band is sustained for >12 h.
Figure 5.
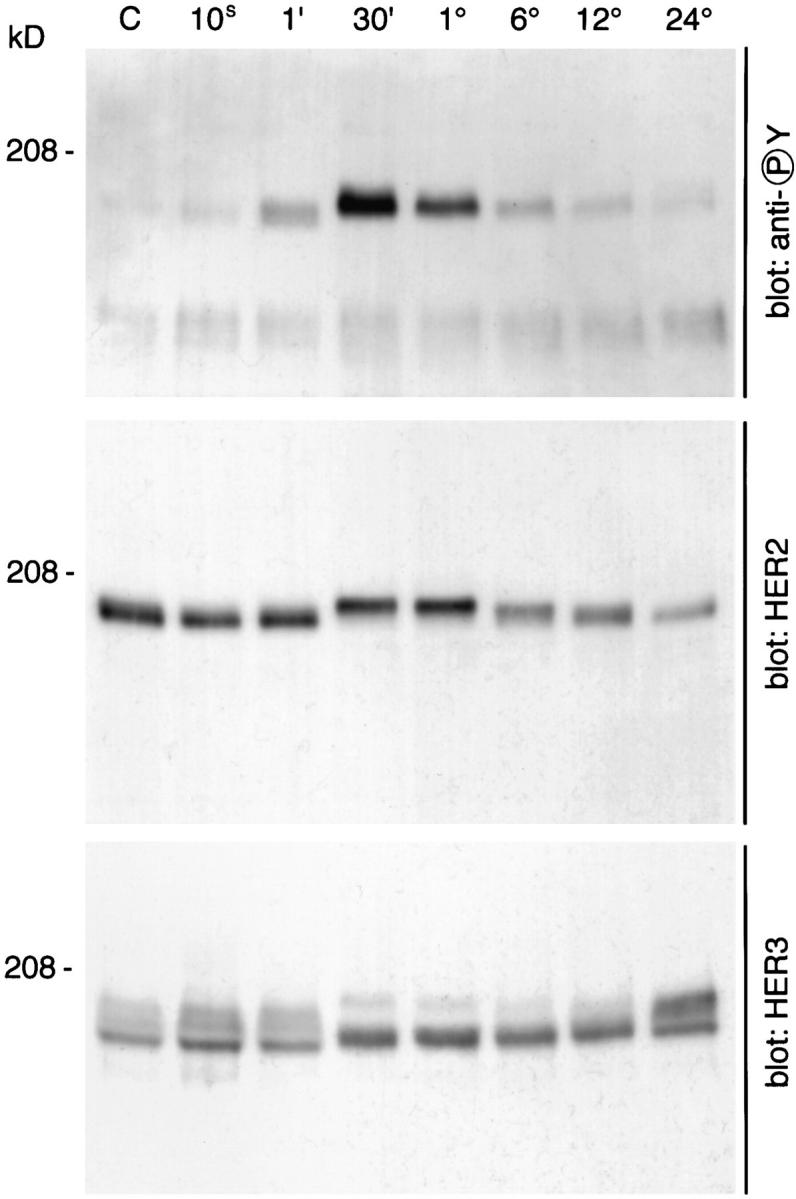
Neuregulin induces a mobility shift in HER2 and HER3 and the rapid phosphorylation of a 180–190-kD protein in Schwann cells. Schwann cells were cultured as described in Materials and Methods, treated with neuregulin for the times indicated (control, 10 s, 1 min, 30 min, 1 h, 6 h, 12 h, and 24 h), and then lysed with ice-cold lysis buffer. Total cell lysates were resolved by 5% PAGE and transferred to PVDF membranes. HER2 and HER3 were visualized with polyclonal antisera, and tyrosine-phosphorylated proteins were visualized with the monoclonal antibody G410.
Activation of neuregulin receptors in Schwann cells was studied in further detail by immunoprecipitation of neuregulin receptors followed by blotting with antiphosphotyrosine antibodies. Recent studies in which total tyrosinephosphorylated proteins were immunoprecipitated from Schwann cells treated with neuregulin demonstrated the phosphorylation of HER2 and HER3 (24). However, such an analysis precludes investigating receptor heterodimerization. In the experiments described below, Schwann cells were treated for 20 min with neuregulin or control buffer (PBS), and individual neuregulin receptors were immunoprecipitated as described in the Materials and Methods. Neuregulin induces the tyrosine phosphorylation of a 180– 190-kD band detected in both HER2 and HER3 immunoprecipitates (Fig. 6 A). We again identified a neuregulin induced mobility shift of ∼5 kD that occurs upon phosphorylation for HER2. Two antibodies were used to examine HER3 on Western blots: a monoclonal antibody that has not been mapped to a specific domain of the receptor and a polyclonal antibody that was raised against a peptide in the COOH terminus of HER3 that contains a consensus sequence for tyrosine phosphorylation. When blots were stripped and reprobed using the HER3 monoclonal antibody, a similar mobility shift was observed as broadening of the HER3 band (Fig. 6 A). This mobility shift was not observed with the HER3 polyclonal antibody because this antibody does not recognize the phosphorylated form of HER3 as well as dephospho-HER3 on Western blots (Fig. 6 A).
Figure 6.
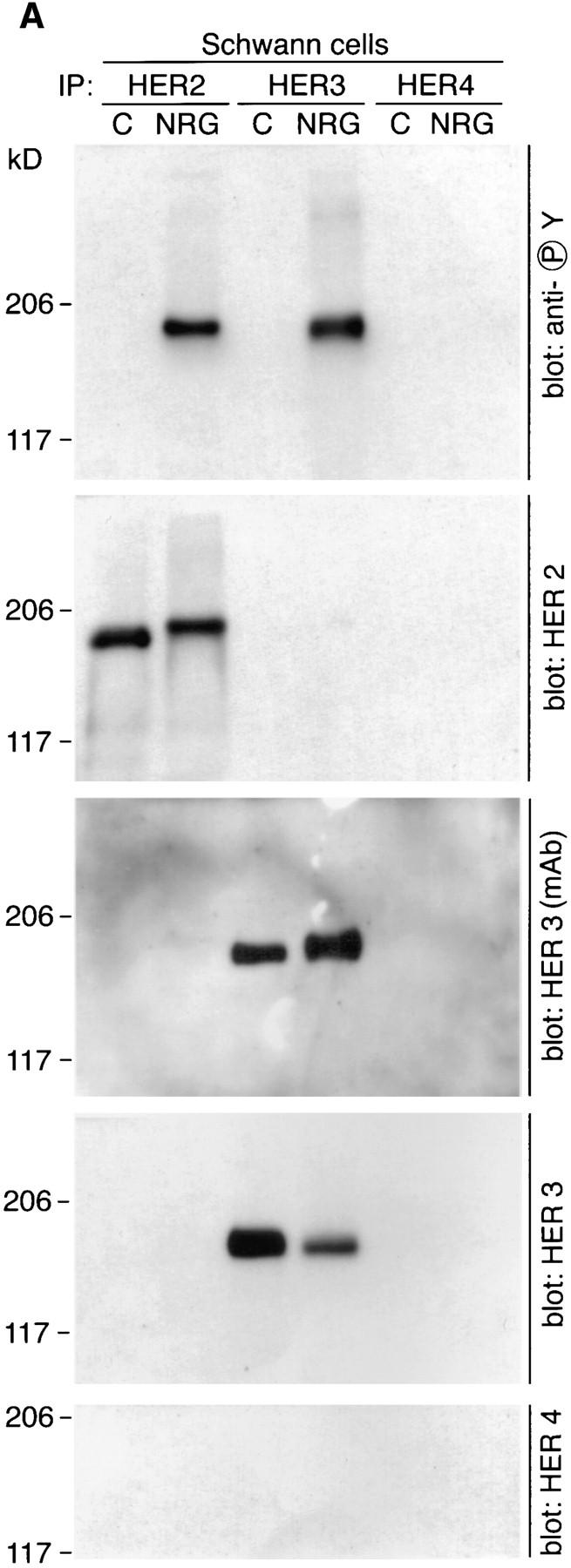
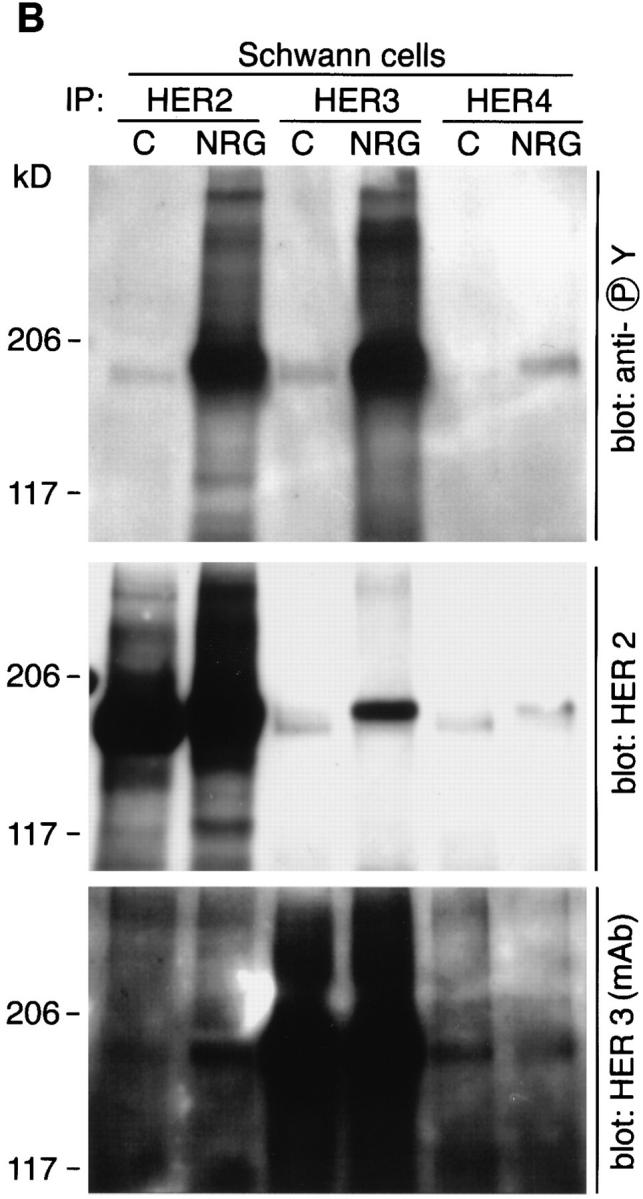
Neuregulin induces the tyrosine phosphorylation of HER2 and HER3, and the formation of HER2:HER3 heterodimers. Schwann cells were treated with a control solution (PBS) or 100 pM neuregulin for 20 min and then lysed in ice-cold lysis buffer. HER2, HER3, and HER4 were immunoprecipitated from lysates as previously described. Immunoprecipitates were resolved by 5% SDS-PAGE, proteins were transferred to PVDF membranes, and membranes were probed with the indicated antibodies.
Prolonged exposure of the above blots shows HER3 immunoreactivity in HER2 immunoprecipitates and HER2 immunoreactivity in HER3 immunoprecipitates from neuregulin-treated but not control Schwann cells (Fig. 6 B). This indicates that neuregulin induces receptor heterodimerization in Schwann cells. We cannot rule out the existence of receptor homodimers as well.
Sensory Neurons Express Multiple Neuregulins That Are Biologically Active
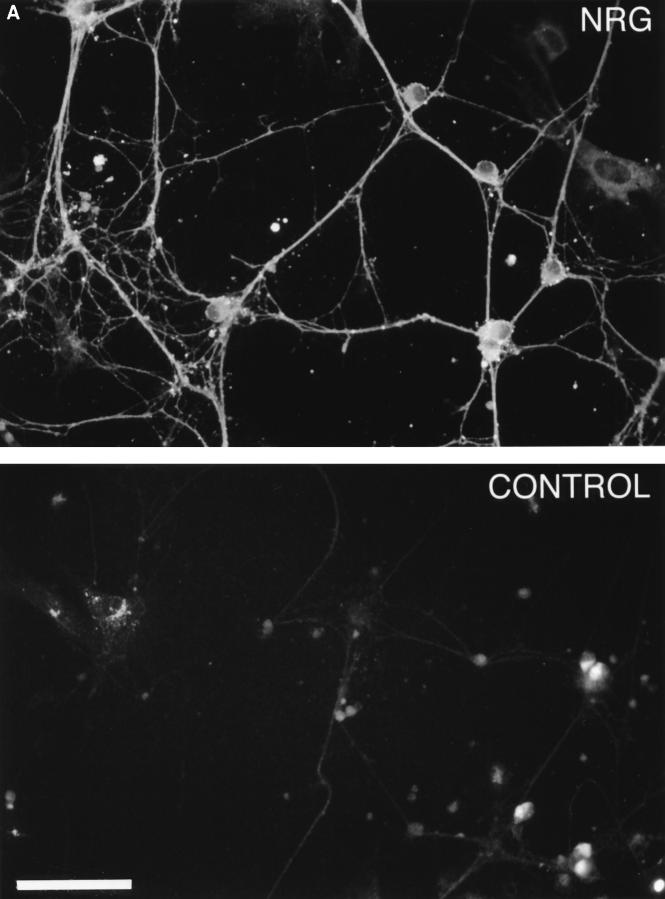
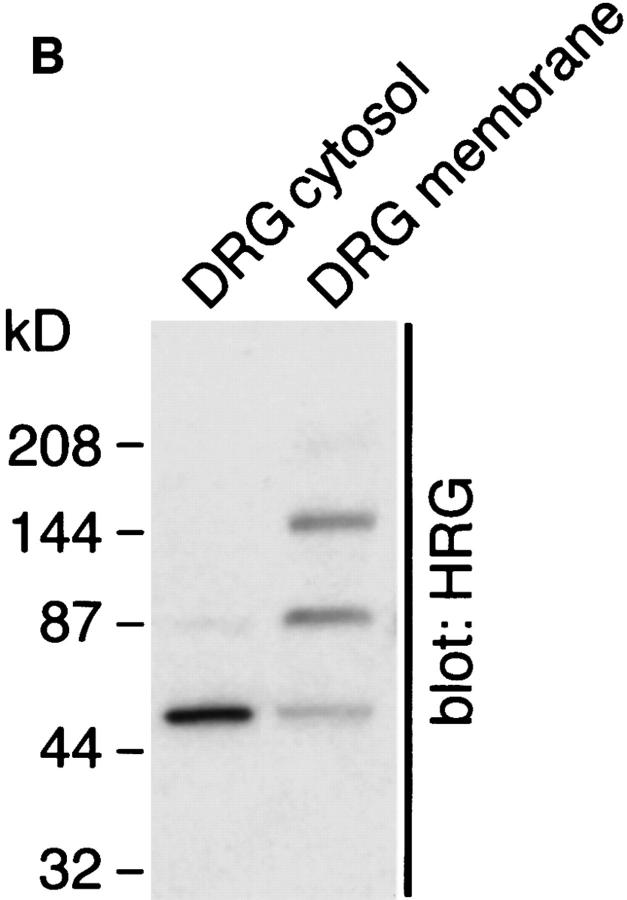
Having established that CNS and PNS glia have distinct neuregulin receptors, we wanted to determine if neurons secreted neuregulins and if they were present on the cell surface. We chose DRG neurons to study because they possess both CNS and PNS myelinated axons. Sensory neurons were obtained from the dorsal root ganglia of embryonic day (E)15 rats and were freed of fibroblasts and Schwann cells by treatment with 5-fluorodeoxyuridine as described in the Materials and Methods section. Neuregulin staining, detected with an antibody recognizing a COOH terminus cytoplasmic domain, is present in both the soma and neurites of cultured DRG neurons (Fig. 7 A). Cultured sensory neurons express three forms of membraneassociated neuregulin by Western blot analysis with apparent molecular masses of 144, 84, and 55 kD (Fig. 7 B). The 84-kD band is consistent with a full-length Ig-domain–containing protein. A neuregulin isoform of ∼144 kD was previously noted in Western blots of rat spinal cord (63). An ∼144-kD isoform can be predicted from known alternative splice sites, but such a cDNA has not been cloned. A 55-kD band was present in both membrane and cytosolic fractions. The 55-kD band in membrane fractions likely represents the remaining transmembrane domain plus the cytosolic domain after extracellular proteolytic processing of full-length neuregulin. In the cytosol, this 55-kD band is the free soluble cytosolic domain, which has not been previously demonstrated as a stable product of neuregulin processing (Fig. 7 B). These results suggests that, in addition to cell surface neuregulins, a proportion of the neuregulins expressed by sensory neurons are processed to release the extracellular domain.
Figure 7.
DRG neurons express multiple neuregulin isoforms. (A) Neurons isolated from E15 DRGs were cultured for 3 wk as described, fixed, and stained with an antibody that recognizes a peptide within the COOH terminus of the neuregulin “a” form. Immunoreactive cells were recognized with Cy3-conjugated secondary antibodies and epifluorescence. Both processes and soma stain intensely for neuregulin. (B) Western blot of cytosolic and membrane fractions from DRG neurons. Neurons isolated from DRGs were cultured for 3 wk and lysed in hypotonic buffer, and membrane and cytosolic fractions were prepared as detailed in Materials and Methods. Proteins were solubilized in SDS-DTT–containing buffer and resolved on 4–15% gradient gels, and the resulting blots were probed with the antineuregulin antibody described above. Bar, 50 μm.
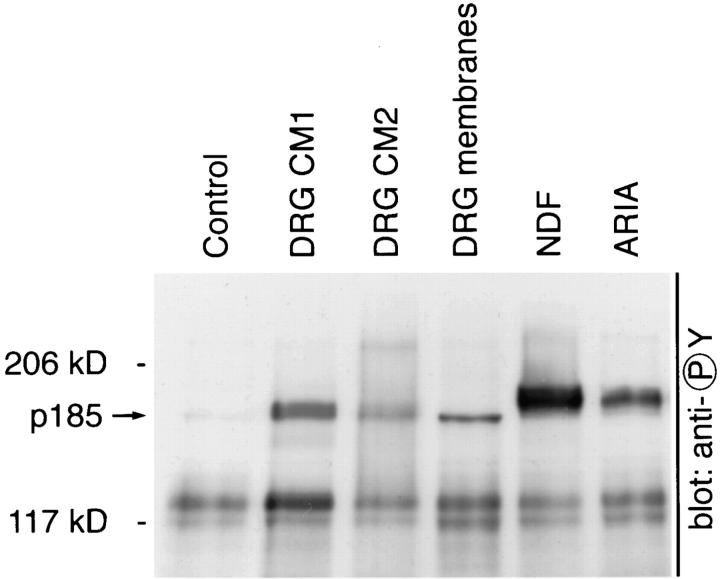
Both neuregulin secreted into the medium and that associated with the cell surface possess biological activity. Concentrated conditioned medium and a preparation of cell membranes from sensory neuron cultures were tested for p185 (neuregulin receptor) tyrosine phosphorylation using L6 muscle cells, which express high levels of neuregulin receptors. The conditioned medium samples stimulated significant p185 phosphorylation, confirming that active neuregulins are released by sensory neurons in culture (Fig. 8). Furthermore, the DRG cell membranes were also positive in this assay, demonstrating that surface bound neuregulins are able to play a role in signaling (Fig. 8).
Figure 8.
Neuregulin from DRG neurons is biologically active. Conditioned medium isolated from two separate cultures of DRG neurons (DRG CM 1 and 2) and membrane vesicles from DRG neurons were prepared as described and studied for their ability to induce tyrosine phosphorylation of p185 in L6 cells. L6 cells were incubated with conditioned medium for 30 min and then harvested in SDS-loading buffer with β-mercaptoethanol. Lysates were resolved on 5% polyacrylamide gels, and proteins were transferred to PVDF membranes that were probed with antiphosphotyrosine antibodies. p185 phosphorylation is evident in L6 cells treated with DRG-conditioned medium, EGFβ1-like domain of neu differentiation factor, and recombinant acetylcholine receptor–inducing activity, but not control medium or fibroblast conditioned medium.
Discussion
Our data provide evidence for unique neuregulin receptor usage in glial cells. Neuregulin signaling in Schwann cells is different from that in O2A and oligodendrocyte cells in a number of respects. HER2 and HER3 are the major receptors on Schwann cells, in agreement with previous reports (24, 34, 45, 46), whereas HER2 and HER4 predominate in oligodendroglial cells. In Schwann cells, no heterodimers were detected in the absence of ligand, whereas in oligodendroglial cells, HER2:HER4 heterodimers were identified. Addition of neuregulins to Schwann cells induced HER2:HER3 heterodimerization and tyrosine phosphorylation of both subunits.
In oligodendroglial cells, no ligand-dependent change in the amount of HER heterodimers was observed, and only HER4 was tyrosine phosphorylated. Although this might suggest that neuregulins signal primarily through HER4 homodimers in cells of the oligodendrocyte lineage, we clearly detected ligand-dependent activation of HER4 in HER2:HER4 heterodimers, suggesting these heterodimers also play a role. The levels of one receptor subunit, HER2, decline in Schwann cells after prolonged exposure to ligand. If the biological outcome in response to neuregulins is due to neuregulin receptor usage, then the phosphorylation of HER2 and/or HER3 may be relevant to a mitogenic response in Schwann cells, and the absence of these phosphorylation events may explain the absence of a mitogenic response in cells of the oligodendrocyte lineage. The presence of phosphorylated HER4 may play a role in signaling differentiation when it is the dominant neuregulin receptor expressed and phosphorylated, as in oligodendrocytes. In addition to differences in neuregulin receptor isotypes between PNS and CNS glia, Schwann cells also express quantitatively more receptor than do CNS glia. Thus, it is possible that the differences in biological effects of neuregulins on Schwann cells and cells in the oligodendrocyte lineage are due to receptor number as well as isotype. While we did not study neuregulin receptor phosphorylation in astrocytes, we did determine that astrocytes express HER2, HER3, and HER4. Neuregulin acts as a survival factor for astrocytes, and it will be interesting to determine if this survival effect is imparted by unique neuregulin receptor usage. The absence of detectable HER3 in our cultures of O2A progenitor cells and oligodendrocytes argues for minimal astrocytic contamination of these cells.
The cellular response of Schwann cells to neuregulins differs depending on the stage of development. Neuregulins restrict neural crest cells to a glial fate at E10.5 (65), promote the survival of Schwann cell precursors at E15–16 (11), and stimulate proliferation of mature Schwann cells at P0–1 (11, 34, 39, 46). Given the apparent dependence of neuregulin responses upon relative HER receptor subunit levels, it will be interesting to compare receptor expression at these different stages and additionally later on during myelination of peripheral nerve.
The task of myelination is accomplished by oligodendrocytes in the CNS and by Schwann cells in the PNS. The intimate relationship between axon and oligodendrocyte or Schwann cell that culminates in formation of the myelin internode is predicted to require specific receptor-mediated events and subsequent intracellular signaling from axon to myelin-forming cell and from myelin-forming cell to axon. The known or inferred signal transducing surface receptors on O2A progenitor cells and oligodendrocytes include the PDGF receptor, FGF receptor, CNTF/LIF receptors, IGF-I receptor, and the retinoic acid receptor (1–3, 14, 16–18, 21, 22, 23, 25, 37, 40, 42–44, 50, 60) in addition to specific glycolipids present on the oligodendrocyte surface thought to play a role in transducing signals (12, 13). However, of these receptors, none have ligands that are known to be expressed on the surface of axons (10, 30, 38, 47–49, 57, 64, 67).
To support the idea that axonal-derived neuregulin acts as a ligand for neuregulin receptors on O2A progenitor cells and Schwann cells, we examined DRG neurons for production of neuregulins. Myelinated axons of DRG neurons exist in peripheral nerve and the dorsal columns in the CNS. Thus, DRG neurons are a reasonable cell to study for axonal-derived factors that may influence both Schwann cells and oligodendrocytes. In conditioned medium from DRG neurons, there is neuregulin activity as determined by assay of receptor phosphorylation, supporting the concept that soluble ligand is released from neurons. Membrane fractions of DRG neurons are also capable of stimulating receptor phosphorylation, and thus we argue that membrane-associated neuregulin is also capable of activating receptors. There are three membrane-associated forms of neuregulin in DRG neurons that we detected with antibodies recognizing a COOH-terminal epitope restricted to the “a”-cytoplasmic domain (71). Two of these membrane-associated forms (100 and 140 kD) represent large transmembrane forms of neuregulin, and the smaller 50-kD membrane-associated form is most likely the intact cytosolic and transmembrane domains minus the extracellular domain. The released, soluble cytosolic domain is clearly present and stable in cytosolic fractions. These data suggest that there may be two processing events for transmembrane forms of neuregulin occurring at the extracellular surface to release ligand into the extracellular spaces, and at the intracellular surface to release the cytosolic domain into the cytosol. The function of the cytosolic domain is unknown, but its importance is implied by being the most conserved domain between different neuregulins.
In our cultures, O2A progenitor cells and oligodendrocyte cells are indistinguishable, both in terms of HER receptors expressed and in terms of the tyrosine phosphorylation response to added neuregulin, suggesting that maturation of this lineage does not alter neuregulin responsiveness. It is interesting to note that O2A progenitor cells that have been expanded six passages in conditioned medium from the B104 cell line (6), akin to conditions used to generate the CG4 glial cell line (36), express HER3 abundantly and have relatively less HER4 (6). The cellular response to neuregulins in these cells is dramatically different to that of primary O2A progenitor cells or oligodendrocyte cells; neuregulins stimulate a robust mitogenic response and arrest differentiation into mature oligodendrocytes (6). We speculate that the expression of high levels of HER3 is responsible for these changes. However, it is possible that other changes in cell cycle control occur with manifold passaging of primary glial cells (32, 55). Future experiments to prove the premise that HER3 specifies a mitogenic signal will involve transfecting cells of the oligodendrocyte lineage with HER3 expression vectors.
Acknowledgments
This work was supported by grants from the National Institute of Neurologic Disorders and Stroke, Nos. RO1NS18458 (G.D. Fischbach), KO8NS01703 (T.K. Vartanian), and NIH-P30-HD18655.
Abbreviations used in this paper
- CNS and PNS
central and peripheral nervous systems
- DRG
dorsal root ganglion
- E
embryonic day
- EGFR
epidermal growth factor receptor
- GGF
glial growth factor
- O2A
oligodendrocyte–type II astrocyte
- P
postnatal day
- PVDF
polyvinyl difluoride
Footnotes
We would like to acknowledge the late Dr. Richard Bunge, who was a pioneer in the fields of Schwann cell biology and axonal glial interactions, and whose contributions form a firm base upon which our current experiments are built. The authors also thank Amgen Biologicals (Thousand Oaks, CA) for providing the recombinant EGFβ1 domain of neuregulin.
Address all correspondence to Dr. Timothy Vartanian, Department of Neurobiology, Harvard Medical School, WAB 328, 200 Longwood Avenue, Boston, MA 02115.
References
- 1.Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- 2.Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development (Camb) 1993;118:283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- 3.Barres BA, Raff MC, Gaese F, Bartke I, Dechant G, Barde YA. A crucial role for neurotrophin-3 in oligodendrocyte development. Nature (Lond) 1994;367:371–375. doi: 10.1038/367371a0. [DOI] [PubMed] [Google Scholar]
- 4.Ben BN, Yarden Y. Neu differentiation factors: a family of alternatively spliced neuronal and mesenchymal factors. Proc Soc Exp Biol Med. 1994;206:221–227. doi: 10.3181/00379727-206-43746. [DOI] [PubMed] [Google Scholar]
- 5.Bunge, M.B., R.P. Bunge, D.J. Carey, C.J. Cornbrooks, C.F. Eldridge, A.K. Williams, and P.M. Wood. 1983. Axonal and nonaxonal influences in Schwann cell development. In Developing and Regenerating Vertebrate Nervous Systems. P. Coates, R. Markwald, and A. Kenny, editors. Alan R. Liss, New York. 71–105.
- 6.Canoll PD, Musacchia JM, Hardy R, Reynolds R, Marchionni M, Salzer JL. GGF/Neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996;17:229–243. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- 7.Carraway K, Burden SJ. Neuregulins and their receptors. Curr Opin Neurobiol. 1995;5:606–612. doi: 10.1016/0959-4388(95)80065-4. [DOI] [PubMed] [Google Scholar]
- 8.Carraway K, Sliwkowski MX, Akita R, Platko JV, Guy PM, Nuijens A, Diamonti AJ, Vandlen RL, Cantley LC, Cerione RA. The erbB3 gene product is a receptor for heregulin. J Biol Chem. 1994;269:14303–14306. [PubMed] [Google Scholar]
- 9.Coussens L, Yang FT, Liao YC, Chen E, Gray A, McGrath J, Seeburg PH, Libermann TA, Schlessinger J, Francke U, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science (Wash DC) 1985;230:1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- 10.DiStefano PS, Friedman B, Radziejewski C, Alexander C, Boland P, Schick CM, Lindsay RM, Wiegand SJ. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- 11.Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen KR. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron. 1995;15:585–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 12.Dyer CA, Matthieu JM. Antibodies to myelin/oligodendrocyte-specific protein and myelin/oligodendrocyte glycoprotein signal distinct changes in the organization of cultured oligodendroglial membrane sheets. J Neurochem. 1994;62:777–787. doi: 10.1046/j.1471-4159.1994.62020777.x. [DOI] [PubMed] [Google Scholar]
- 13.Dyer CA, Philibotte TM, Wolf MK, Billings GS. Myelin basic protein mediates extracellular signals that regulate microtubule stability in oligodendrocyte membrane sheets. J Neurosci Res. 1994;39:97–107. doi: 10.1002/jnr.490390112. [DOI] [PubMed] [Google Scholar]
- 14.Ellison, J.A., and V.J. de. 1994. Platelet-derived growth factor receptor is expressed by cells in the early oligodendrocyte lineage. J. Neurosci. Res. 37:116–128. [DOI] [PubMed]
- 15.Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- 16.Gallo V, Armstrong RC. Developmental and growth factorinduced regulation of nestin in oligodendrocyte lineage cells. J Neurosci. 1995;15:394–406. doi: 10.1523/JNEUROSCI.15-01-00394.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gard AL, Pfeiffer SE. Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development (Camb) 1989;106:119–132. doi: 10.1242/dev.106.1.119. [DOI] [PubMed] [Google Scholar]
- 18.Gard AL, Pfeiffer SE. Glial cell mitogens bFGF and PDGF differentially regulate development of O4+GalC−oligodendrocyte progenitors. Dev Biol. 1993;159:618–630. doi: 10.1006/dbio.1993.1269. [DOI] [PubMed] [Google Scholar]
- 19.Goldman JE. Regulation of oligodendrocyte differentiation. Trends Neurosci. 1992;15:359–362. doi: 10.1016/0166-2236(92)90179-c. [DOI] [PubMed] [Google Scholar]
- 20.Goodearl AD, Davis JB, Mistry K, Minghetti L, Otsu M, Waterfield MD, Stroobant P. Purification of multiple forms of glial growth factor. J Biol Chem. 1993;268:18095–18102. [PubMed] [Google Scholar]
- 21.Grinspan J, Wrabetz L, Kamholz J. Oligodendrocyte maturation and myelin gene expression in PDGF-treated cultures from rat cerebral white matter. J Neurocytol. 1993;22:322–333. doi: 10.1007/BF01195556. [DOI] [PubMed] [Google Scholar]
- 22.Grinspan JB, Franceschini B. Platelet-derived growth factor is a survival factor for PSA-NCAM+ oligodendrocyte pre-progenitor cells. J Neurosci Res. 1995;41:540–551. doi: 10.1002/jnr.490410414. [DOI] [PubMed] [Google Scholar]
- 23.Grinspan JB, Stern JL, Pustilnik SM, Pleasure D. Cerebral white matter contains PDGF-responsive precursors to O2A cells. J Neurosci. 1990;10:1866–1873. doi: 10.1523/JNEUROSCI.10-06-01866.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grinspan JB, Marchionni MA, Reeves M, Coulaloglou M, Scherer SS. Axonal interactions regulate Schwann cell apoptosis in the developing peripheral nerve: neuregulin receptors and the role of neuregulins. J Neurosci. 1996;16:6107–6118. doi: 10.1523/JNEUROSCI.16-19-06107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart IK, Richardson WD, Heldin CH, Westermark B, Raff MC. PDGF receptors on cells of the oligodendrocyte-type-2 astrocyte (O-2A) cell lineage. Development (Camb) 1989;105:595–603. doi: 10.1242/dev.105.3.595. [DOI] [PubMed] [Google Scholar]
- 26.Holmes WE, Sliwkowski MX, Akita RW, Henzel WJ, Lee J, Park JW, Yansura D, Abadi N, Raab H, Lewis GD, et al. Identification of heregulin, a specific activator of p185erbB2. Science (Wash DC) 1992;256:1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- 27.Huang CL, Keynes RJ. Terminal sprouting of mouse motor nerves when the post-synaptic membrane degenerates. Brain Res. 1983;274:225–229. doi: 10.1016/0006-8993(83)90699-6. [DOI] [PubMed] [Google Scholar]
- 28.Jacobsen NE, Abadi N, Sliwkowski MX, Reilly D, Skelton NJ, Fairbrother WJ. High-resolution solution structure of the EGFlike domain of heregulin-alpha. Biochemistry. 1996;35:3402–3417. doi: 10.1021/bi952626l. [DOI] [PubMed] [Google Scholar]
- 29.Kita YA, Barff J, Luo Y, Wen D, Brankow D, Hu S, Liu N, Prigent SA, Gullick WJ, Nicolson M. NDF/heregulin stimulates the phosphorylation of Her3/erbB3. FEBS (Fed Exp Biol Soc) Lett. 1994;349:139–143. doi: 10.1016/0014-5793(94)00644-x. [DOI] [PubMed] [Google Scholar]
- 29a.Kleitman, N., P.W. Wood, and R.P. Bunge. 1994. Culturing Nerve Cells. Gary Banker and Kimberly Goslin, editors. MIT Press, Cambridge, MA. 337–377.
- 30.Kostyk SK, D'Amore PA, Herman IM, Wagner JA. Optic nerve injury alters basic fibroblast growth factor localization in the retina and optic tract. J Neurosci. 1994;14:1441–1449. doi: 10.1523/JNEUROSCI.14-03-01441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraus MH, Issing W, Miki T, Popescu NC, Aaronson SA. Isolation and characterization of ERBB3, a third member of the ERBB/ epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc Natl Acad Sci USA. 1989;86:9193–9197. doi: 10.1073/pnas.86.23.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langford LA, Porter S, Bunge RP. Immortalized rat Schwann cells produce tumours in vivo. J Neurocytol. 1988;17:521–529. doi: 10.1007/BF01189807. [DOI] [PubMed] [Google Scholar]
- 33.Lemke GE, Brockes JP. Identification and purification of glial growth factor. J Neurosci. 1984;4:75–83. doi: 10.1523/JNEUROSCI.04-01-00075.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levi AD, Bunge RP, Lofgren JA, Meima L, Hefti F, Nikolics K, Sliwkowski MX. The influence of heregulins on human Schwann cell proliferation. J Neurosci. 1995;15:1329–1340. doi: 10.1523/JNEUROSCI.15-02-01329.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loeb JA, Fischbach GD. ARIA can be released from extracellular matrix through cleavage of a heparin-binding domain. J Cell Biol. 1995;130:127–135. doi: 10.1083/jcb.130.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louis JC, Magal E, Muir D, Manthorpe M, Varon S. CG-4, a new bipotential glial cell line from rat brain, is capable of differentiating in vitro into either mature oligodendrocytes or type-2 astrocytes. J Neurosci Res. 1992;31:193–204. doi: 10.1002/jnr.490310125. [DOI] [PubMed] [Google Scholar]
- 37.Louis JC, Magal E, Takayama S, Varon S. CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science (Wash DC) 1993;259:689–692. doi: 10.1126/science.8430320. [DOI] [PubMed] [Google Scholar]
- 38.Ma J, Yang SX, Ho GJ, Festoff BW. Insulin-like growth factor binding protein-1 is pre-synaptic at mouse neuromuscular synapses and is transported in nerve. Neurochem Res. 1994;19:1363–1368. doi: 10.1007/BF00972464. [DOI] [PubMed] [Google Scholar]
- 39.Marchionni MA, Goodearl AD, Chen MS, Bermingham MO, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K, et al. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature (Lond) 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 40.McKinnon RD, Piras G, Ida JJ, Dubois DM. A role for TGF-β in oligodendrocyte differentiation. J Cell Biol. 1993;121:1397–1407. doi: 10.1083/jcb.121.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKinnon RD, Smith C, Behar T, Smith T, Dubois DM. Distinct effects of bFGF and PDGF on oligodendrocyte progenitor cells. Glia. 1993;7:245–254. doi: 10.1002/glia.440070308. [DOI] [PubMed] [Google Scholar]
- 42.McMorris FA, Dubois DM. Insulin-like growth factor I promotes cell proliferation and oligodendroglial commitment in rat glial progenitor cells developing in vitro. J Neurosci Res. 1988;21:199–209. doi: 10.1002/jnr.490210212. [DOI] [PubMed] [Google Scholar]
- 43.McMorris FA, Furlanetto RW, Mozell RL, Carson MJ, Raible DW. Regulation of oligodendrocyte development by insulin-like growth factors and cyclic nucleotides. Ann NY Acad Sci. 1990;605:101–109. doi: 10.1111/j.1749-6632.1990.tb42385.x. [DOI] [PubMed] [Google Scholar]
- 44.McMorris FA, Mozell RL, Carson MJ, Shinar Y, Meyer RD, Marchetti N. Regulation of oligodendrocyte development and central nervous system myelination by insulin-like growth factors. Ann NY Acad Sci. 1993;692:321–334. doi: 10.1111/j.1749-6632.1993.tb26247.x. [DOI] [PubMed] [Google Scholar]
- 45.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature (Lond) 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 46.Morrissey TK, Levi AD, Nuijens A, Sliwkowski MX, Bunge RP. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc Natl Acad Sci USA. 1995;92:1431–1435. doi: 10.1073/pnas.92.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mudhar HS, Pollock RA, Wang C, Stiles CD, Richardson WD. PDGF and its receptors in the developing rodent retina and optic nerve. Development (Camb) 1993;118:539–552. doi: 10.1242/dev.118.2.539. [DOI] [PubMed] [Google Scholar]
- 48.Nieto BM, Garcia SL, Torres AI. Orthograde transport and release of insulin-like growth factor I from the inferior olive to the cerebellum. J Neurosci Res. 1993;36:520–527. doi: 10.1002/jnr.490360504. [DOI] [PubMed] [Google Scholar]
- 49.Nishio T, Furukawa S, Akiguchi I, Oka N, Ohnishi K, Tomimoto H, Nakamura S, Kimura J. Cellular localization of nerve growth factor-like immunoreactivity in adult rat brain: quantitative and immunohistochemical study. Neuroscience. 1994;60:67–84. doi: 10.1016/0306-4522(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 50.Noll E, Miller RH. Regulation of oligodendrocyte differentiation: a role for retinoic acid in the spinal cord. Development (Camb) 1994;120:649–660. doi: 10.1242/dev.120.3.649. [DOI] [PubMed] [Google Scholar]
- 51.Orr UA, Trakhtenbrot L, Ben LR, Wen D, Rechavi G, Lonai P, Yarden Y. Neural expression and chromosomal mapping of Neu differentiation factor to 8p12-p21. Proc Natl Acad Sci USA. 1993;90:1867–1871. doi: 10.1073/pnas.90.5.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peles E, Yarden Y. Neu and its ligands: from an oncogene to neural factors. BioEssays. 1993;15:815–824. doi: 10.1002/bies.950151207. [DOI] [PubMed] [Google Scholar]
- 53.Peles E, Ben LR, Tzahar E, Liu N, Wen D, Yarden Y. Celltype specific interaction of Neu differentiation factor (NDF/heregulin) with Neu/HER-2 suggests complex ligand-receptor relationships. EMBO (Eur Mol Biol Organ) J. 1993;12:961–971. doi: 10.1002/j.1460-2075.1993.tb05737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plowman GD, Culouscou JM, Whitney GS, Green JM, Carlton GW, Foy L, Neubauer MG, Shoyab M. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci USA. 1993;90:1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porter S, Glaser L, Bunge RP. Release of autocrine growth factor by primary and immortalized Schwann cells. Proc Natl Acad Sci USA. 1987;84:7768–7772. doi: 10.1073/pnas.84.21.7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qian X, LeVea CM, Freeman JK, Dougall WC, Greene MI. Heterodimerization of epidermal growth factor receptor and wildtype or kinase-deficient Neu: a mechanism of interreceptor kinase activation and transphosphorylation. Proc Natl Acad of Sci USA. 1994;91:1500–1504. doi: 10.1073/pnas.91.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quiroga S, Garofalo RS, Pfenninger KH. Insulin-like growth factor I receptors of fetal brain are enriched in nerve growth cones and contain a β-subunit variant. Proc Natl Acad Sci USA. 1995;92:4309–4312. doi: 10.1073/pnas.92.10.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raff MC, Abney E, Brockes JP, Hornby-Smith A. Schwann cell growth factors. Cell. 1978;15:813–822. doi: 10.1016/0092-8674(78)90266-0. [DOI] [PubMed] [Google Scholar]
- 59.Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature (Lond) 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- 60.Raff MC, Lillien LE, Richardson WD, Burne JF, Noble MD. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature (Lond) 1988;333:562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- 61.Ratner, N., P. Wood, L. Glaser, and R.P. Bunge. 1987. Further characterization of the neuronal cell surface protein mitogenic for Schwann cells. In Glial-Neuronal Communication in Development and Regeneration. H.H. Althaus and W. Seifert, editors. Springer-Verlag, Heidelberg. 685–697.
- 62.Salzer JL, Bunge RP. Studies of Schwann cell proliferation I. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J Cell Biol. 1980;84:739–752. doi: 10.1083/jcb.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sandrock AJ, Goodearl AD, Yin QW, Chang D, Fischbach GD. ARIA is concentrated in nerve terminals at neuromuscular junctions and at other synapses. J Neurosci. 1995;15:6124–6136. doi: 10.1523/JNEUROSCI.15-09-06124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- 65.Shah NM, Marchionni MA, Isaacs I, Stroobant P, Anderson DJ. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell. 1994;77:349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 66.Sliwkowski MX, Schaefer G, Akita RW, Lofgren JA, Fitzpatrick VD, Nuijens A, Fendly BM, Cerione RA, Vandlen RL, Carraway KL., III Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J Biol Chem. 1994;269:14661–14665. [PubMed] [Google Scholar]
- 67.Torriglia A, Jeanny JC, Blanquet PR. Immunohistochemical analysis of fibroblast growth factor receptor in bovine retina. Neurosci Lett. 1994;172:125–128. doi: 10.1016/0304-3940(94)90678-5. [DOI] [PubMed] [Google Scholar]
- 68.Vartanian T, Corfas G, Li Y, Fischbach GD, Stefansson K. A role for the acetylcholine receptor-inducing protein ARIA in oligodendrocyte development. Proc Natl Acad Sci USA. 1994;91:11626–11630. doi: 10.1073/pnas.91.24.11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wallasch C, Weiss FU, Niederfellner G, Jallal B, Issing W, Ullrich A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO (Eur Mol Biol Organ) J. 1995;14:4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wen D, Peles E, Cupples R, Suggs SV, Bacus SS, Luo Y, Trail G, Hu S, Silbiger SM, Levy RB, et al. Neu differentiation factor: a transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992;69:559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- 71.Wen D, Suggs SV, Karunagaran D, Liu N, Cupples RL, Luo Y, Janssen AM, Ben BN, Trollinger DB, Jacobsen VL, et al. Structural and functional aspects of the multiplicity of Neu differentiation factors. Mol Cell Biol. 1994;14:1909–1919. doi: 10.1128/mcb.14.3.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolswijk G, Noble M. Cooperation between PDGF and FGF converts slowly dividing O-2A adult progenitor cells to rapidly dividing cells with characteristics of O-2A perinatal progenitor cells. J Cell Biol. 1992;118:889–900. doi: 10.1083/jcb.118.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wood P, Bunge RP. Evidence that sensory axons are mitogenic for Schwann cells. Nature (Lond) 1975;256:662–664. doi: 10.1038/256662a0. [DOI] [PubMed] [Google Scholar]