Abstract
Although the involvement of viruses in alterations of testicular function and in sexually transmitted diseases is well known, paradoxically, the testicular antiviral defense system has virtually not been studied. The well known antiviral activity of interferons (IFNs) occurs via the action of several IFN-induced proteins, among which the 2′5′ oligoadenylate synthetase (2′5′ A synthetase), the double-stranded RNA-activated protein kinase (PKR), and the Mx proteins are the best known. To explore the antiviral capacity of the testis and to study the testicular action of IFNs, we looked for the presence and regulation of these three proteins in isolated seminiferous tubule cells, cultured in the presence or in the absence of IFN α, IFN γ, or Sendai virus. In all conditions tested, the meiotic pachytene spermatocytes and the post-meiotic early spermatids lacked 2′5′ A synthetase, PKR, and Mx mRNAs and proteins. In contrast, Sertoli cells constitutively expressed these mRNAs and proteins, and their levels were greatly increased after IFN α or Sendai virus exposure. While peritubular cells were also able to markedly express 2′5′ A synthetase, PKR, and Mx mRNA and proteins after IFN α or viral exposure, only PKR was constitutively present in these cells. Interestingly, IFN γ had no effect on peritubular cells' 2′5′ A synthetase and Mx production but it enhanced Mx proteins in Sertoli cells. In conclusion, this study reveals that the seminiferous tubules are particularly well equipped to react to a virus attack. The fact that the two key tubular elements of the blood–testis barrier, namely, Sertoli and peritubular cells, were found to assume this protection allows the extension of the concept of blood–testis barrier to the testicular antiviral defense.
The mammalian testis encompasses two main compartments: the interstitium that contains Leydig cells, responsible for testosterone production, and the blood and lymphatic vessels; and the seminiferous tubules, which are bordered by the peritubular myoid cells and composed of the different generations of germ cells associated with the somatic Sertoli cells (Jégou, 1993).
Various noxious environmental agents are known to affect the integrity of male reproductive function, and there is currently much concern about a possible secular deterioration in human and wildlife reproductive health related to the degradation of the environment (Toppari et al., 1996). The environmental agents known to be able to alter male reproductive health may be physical or chemical but also biological, i.e., microorganisms and viruses (Jégou, 1996). The latter occupy a particular place, considering the serious concerns generated by the present propagation of several sexually transmissible viral diseases and their dramatic consequences. Thus, recently, it was revealed that human immunodeficiency virus (HIV)1 infection is greatly deleterious to the male reproductive function, as HIV- infected men are often oligospermic if not azoospermic (Alexander, 1990), and it was shown that HIV can be present in spermatogonia and spermatocytes (Nuovo et al., 1994), as well as in spermatozoa (Baccetti et al., 1994). In addition to HIV, it has long been known that other viruses can be deleterious to the spermatogenic process. For example, orchitis is relatively frequent in case of mumps infection (Mikuz and Damjanov, 1982) and often leads to azoospermia (Scott, 1960).
Surprisingly, in spite of the dramatic effects of certain viruses on testicular function and of the fact that semen represents an essential vector for the transmission of viral infections, very little is known about the ability of the testis to react to a virus attack.
As a first step in the exploration of the testicular antiviral defense system, in a recent study, we investigated interferon (IFN) α and γ expression within rat seminiferous tubules (Dejucq et al., 1995), as IFNs are a well known family of proteins involved in the cellular antiviral defense system (Sen and Lengyel, 1992). We showed that peritubular and Sertoli cells are able to produce high amounts of type I IFN in response to Sendai virus infection, whereas the low basal levels of type I IFNs secreted by germ cells are not modified or only marginally after exposure to this virus. Interestingly, aberrant IFN expression in the transgenic mice testis leads to sterility (Hekman et al., 1988; Iwakura et al., 1988), which may represent one of the mechanisms by which some viral infections result in azoospermia.
Binding of IFNs to specific cell surface receptors is known to induce the synthesis of a large variety of proteins, among which three different gene products, namely 2′5′ oligoadenylate synthetase (2′5′ A synthetase), double-stranded RNA-activated protein kinase (PKR), and Mx protein have attracted much attention, due to their ability to generate an antiviral state (Staeheli, 1990; Samuel, 1991). In the present study, to search for testicular expression of these three IFN-induced anti-viral proteins, Sertoli cells, peritubular cells, and germ cells were isolated and cultured in the presence of IFN α, IFN γ, or Sendai virus, a powerful IFN inducer belonging to the same family as the Mumps virus, and 2′5′ A synthetase, PKR, and Mx were studied at the protein and mRNA level using a range of different techniques. We show that, while germ cells are unable to produce these antiviral proteins in any experimental conditions, Sertoli cells, in contrast to peritubular cells, are constitutive producers of 2′5′ A synthetase, PKR, and Mx2/ Mx3 proteins. We also demonstrate that Sertoli cells and peritubular cells are able to produce high amounts of 2′5′ A synthetase, PKR, Mx1, and Mx2/Mx3 in response to IFN α or to viral exposure, thereby providing the first direct clues of the existence of a testicular anti-viral defense system.
Materials and Methods
Animals and Reagents
Male Sprague-Dawley rats were purchased from Elevage Janvier (Le Genest Saint Isle, Laval, France). Rat-recombinant IFN α (1–2 × 108 U/ mg) γ (4 × 106 U/mg) were generously provided by Dr. van der Meide (TNO Center, Rijswijk, the Netherlands) and Sendai virus kindly provided by Dr. Ruffault (CHR Pontchaillou, Rennes, France).
Cell Lines
Mouse 3T3 and L929 cells were grown in RPMI 1640 with 10% FCS. 3T3 mouse cells transformed with rat Mx1 cDNA and the pSV2-neo plasmid (Staeheli et al., 1986) and 3T3 cells only transformed with the pSV2-neo plasmid were generously provided by Dr. Koch (Albert Ludwigs Universität, Freiburg, Germany). These two cell lines were grown in DME supplemented with G418 (0.5 mg/ml) and 10% FCS.
Antibodies
The mouse monoclonal 2C12 antibody and the rabbit polyclonal antibodies directed against mouse Mx proteins were generously provided by Pr. Haller (Albert Ludwigs Universität; Staeheli and Haller, 1985). Rabbit polyclonal antibodies against mouse PKR were kindly given by Dr. Chen (Massachusetts Institute of Technology; Petreshyn et al., 1988). Commercial rabbit polyclonal antibody against a peptide corresponding to amino acids 489–508 mapping within the carboxy-terminal domain of PKR of mouse origin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Sertoli Cell Preparation
The isolation of Sertoli cells from 20-d-old rats was carried out as described by Skinner and Fritz (1985). The cells, containing 2% of contaminating germ cells and <2% of peritubular cells (Pineau et al., 1991), were seeded at a density of 1.5 × 106 cells/ml in Ham's F-12/DME (vol/vol) supplemented with insulin (10 μg/ml), transferrin (5 μg/ml), and gentamycin (50 μg/ml) and incubated at 32°C in a humidified atmosphere with 5% CO2 and 95% air. On the second day of culture, germ cell contaminants were removed by a brief hypotonic treatment (Galdieri et al., 1981). After this hypotonic treatment, Sertoli cells were contaminated with <2% germ cells. On day 4 of culture, some of the cells were used as controls, whereas the others were treated for 14 h with IFN α (50, 275, and 500 U/ml) or IFN γ (10 and 100 units/ml) or for 28 h with Sendai virus (100 and 500 hemagglutinant units/ml). These IFN concentrations were chosen according to the maximal levels of IFN produced by Sertoli cells (Dejucq et al., 1995).
Peritubular Cell Preparation
Peritubular cells were isolated from 20-d-old rats, according to the method described by Skinner and Fritz (1985). The cells were cultured at 32°C in Ham's F-12/DME supplemented with 10% FCS and became confluent after 8 d of culture. After only 3 d in culture, with daily changes of media, purity of these cells was ∼96%, as assessed by the alcaline phosphatase method (Chapin et al., 1987). Furthermore, no contamination by Leydig cells or macrophages could be detected using a 3β hydroxysteroid dehydrogenase staining technique and an immunocytological method using the mabED2 (Serotec, Innotest, France), respectively. These peritubular cells were then passaged twice. At confluence, they were washed with culture medium without FCS, incubated for 1 d with this medium, and treated as described above for Sertoli cells.
Germ Cell Preparation
Testes from 90-d-old rats were trypsinized and then fractionated by centrifugal elutriation using a rotor (JE-6; Beckman Instruments, Palo Alto, CA). Enrichment of the pachytene spermatocyte and early spermatid fractions obtained was >90% (Pineau et al., 1993). These were cultured at 32°C at a density of 2.5 and 8 × 106 cells/ml as previously described (Pineau et al., 1993) and treated as indicated above for Sertoli cells.
RNA Extraction
Total RNAs were extracted from the different cellular preparations by guanidium-thiocyanate gradient, using the method of Raymondjean et al. (1983).
Northern Blot Analysis
20 μg of total RNA was denatured in formamide–formaldehyde buffer, electrophoresed in a 2% formaldehyde–1% agarose gel, and then transferred onto a Nylon membrane and UV crosslinked. The membranes (Genescreen; Dupont NEN, Boston, MA) were hybridized with the appropriate [α-32P]dCTP cDNA probe labeled by random priming (1.5 × 106 cpm/ml), as previously described (Feinberg and Vogelstein, 1993). Autoradiography was performed by exposure overnight to Kodak X-Omat film at −80°C, in the presence of intensifying screens. To correct for differences in RNA amounts, blots were stripped and rehybridized with a radiolabeled cDNA probe for rat actin.
Molecular Probes.
The cDNA encoding for mouse 2′5′ A synthetases (clones 9-2 and 3-9), the L3 cDNA, encoding for the 40-kD form of mouse 2′5′ A synthetase, the cDNA encoding for the mouse PKR and the cDNA encoding for the murine Mx were kindly provided by Dr. Sen (Cleveland Clinic Foundation, Cleveland, Ohio; Ghosh et al., 1991), Dr. Coccia (Instituto Superiore di Sanita, Rome, Italy; Coccia et al., 1990), Dr. Williams (Cleveland Clinic Foundation; Feng et al., 1992), and Pr. Weissman (Universität Zurick, Switzerland; Staeheli et al., 1986), respectively.
Assay of 2′5′ A Synthetase
Assay of 2′5′ A synthetase was performed as previously described (Chousterman et al., 1987). Results are expressed as units of 2′5′ A synthetase activity per 100 μg of DNA. A positive control (rat lymphocytes stimulated with IFN α) and an internal reference were included in each assay.
Protein Kinase Assay
We purified PKR using poly (I) poly (C) agarose, according to Salzberg et al. (1995). PKR activity was measured using the capacity of PKR to autophosphorylate (Petreshyn et al., 1988). Samples were analyzed on 7.5% polyacrylamide gels containing SDS, and the phosphorylated proteins were detected by autoradiography.
Immunoprecipitation of Mx Proteins
4 h before the end of the different stimulations, the cells were labeled with medium containing 0.1 mCi/ml of [35S]methionine and [35S]cysteine (TRAN35 S-LABELTM; ICN Biomedicals, Costa Mesa, CA). Immunoprecipitation was carried out as previously described (Staeheli et al., 1985). Samples were electrophoresed on a 7.5% polyacrylamide gel containing SDS, and radioactive proteins were visualized by fluorography with Amplify (Amersham Intl., Little Chalfont, UK).
Immunocytochemistry
Immunocytochemistry was performed as described by Meier et al. (1988) on cells cultured in 8-well Lab-Tek chambers (NUNC, Roskilde, Denmark) and using the Universal LSAB 2 KIT (DAKO, Trappes, France) to label the primary antibody. For Mx immunocytochemistry, a mouse monoclonal antibody against mouse Mx proteins, also known to interact with the three rat Mx proteins (antibody 2C12, kindly provided by Pr. Haller), diluted at 1:10, was incubated with the cells for 15 min (Meier et al., 1988). For PKR immunocytochemistry, a commercial rabbit polyclonal antibody directed against mouse PKR (Santa Cruz Biotechnology) was used at the dilution 1:500 for 30 min. Since there was no antibody available against mouse or rat 2′5′ A synthetase, the immunolocalization of 2′5′ A synthetases could not be performed. Control mouse and rabbit IgG were used, respectively, at the same dilutions to ensure the specificity of the labeling.
Results
2′5′ A Synthetase Expression within the Seminiferous Tubule
Northern Blot Experiments.
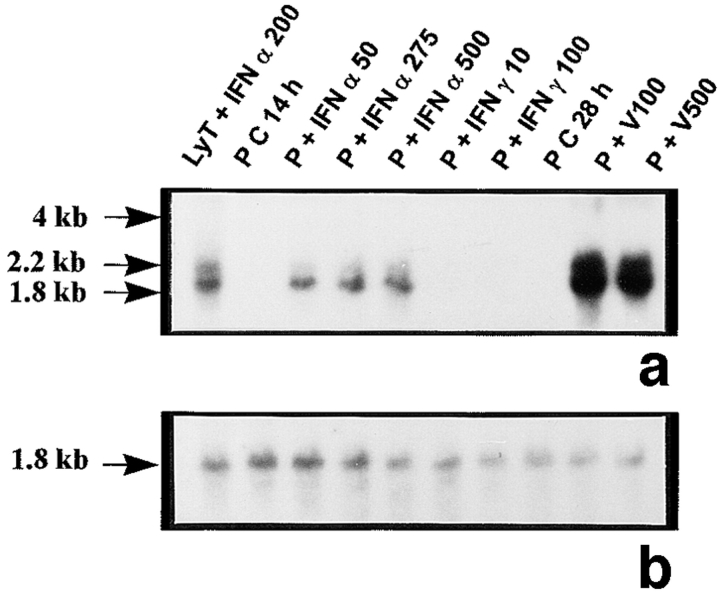
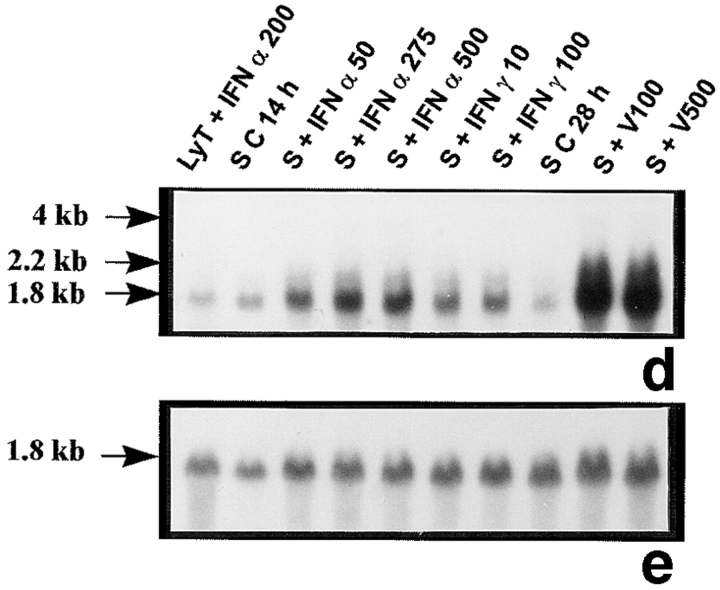
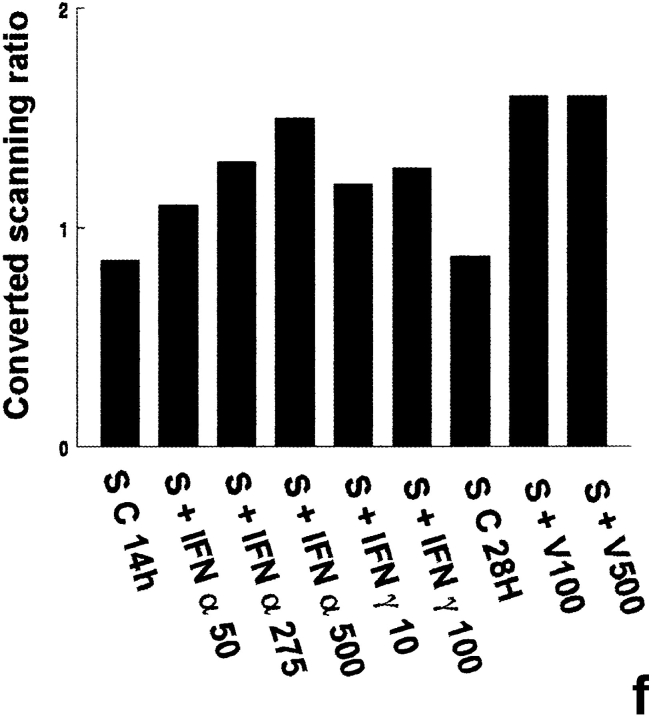
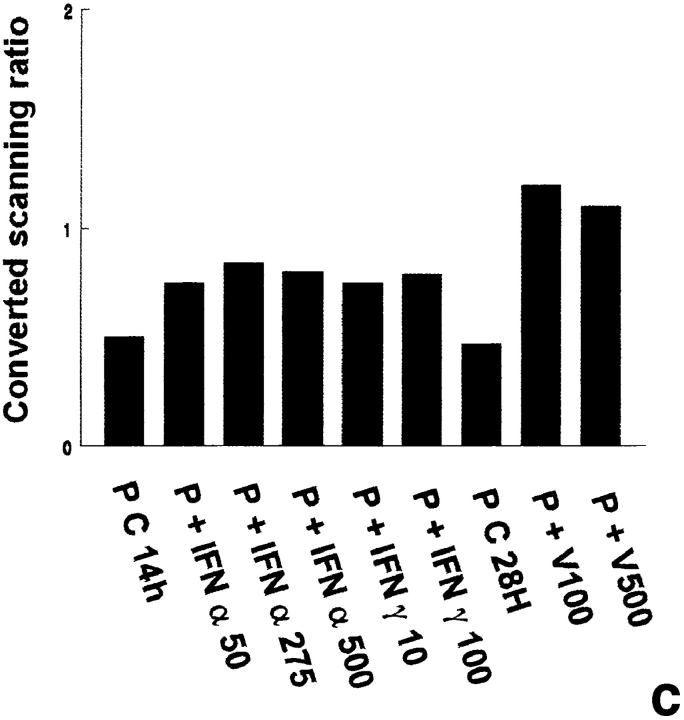
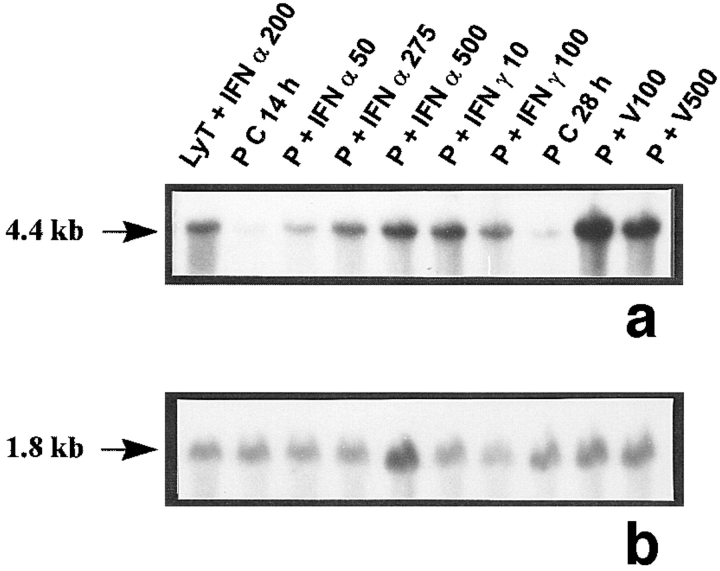
The probes L1, L2, L3, 3-9, and 9-2, coding for different forms of 2′5′ A synthetase in mouse, gave the same results: they all recognized three forms of 2′5′ A synthetase. The results obtained with L3 show that a predominant transcript of 1.8 kb and another less represented transcript of 2.2 kb were visualized in rat lymphocytes stimulated with IFN α used as positive controls (Fig. 1 a). These two latter transcripts were also present in peritubular (Fig. 1 a) and Sertoli cells (Fig. 1 d) stimulated with Sendai virus and, to a lesser extent, in control Sertoli cells (Fig. 1 d). In some experiments, very faint traces of a 4-kb transcript, also observed in mouse L929 cells stimulated with Sendai virus (data not shown), could be seen in Sertoli and peritubular cells stimulated with the Sendai virus. In contrast, neither basal or IFN γ–stimulated peritubular cells (Fig. 1 a) nor germ cells exposed or not exposed to the Sendai virus expressed 2′5′ A synthetase mRNAs (Table I).
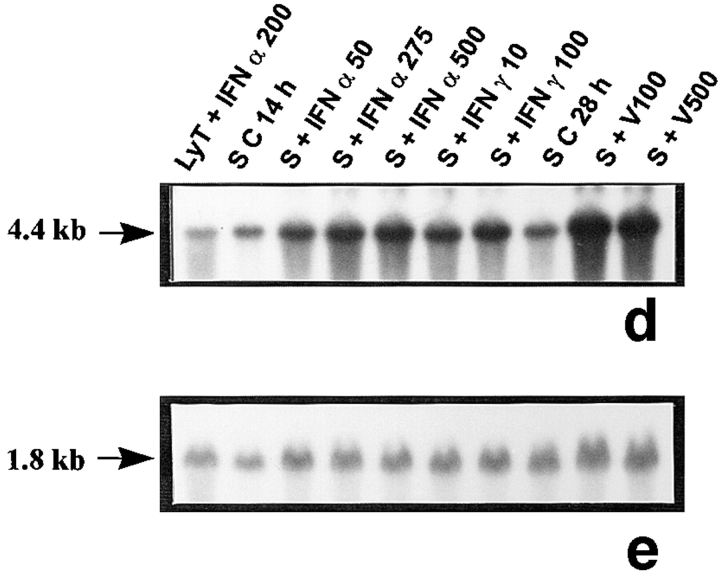
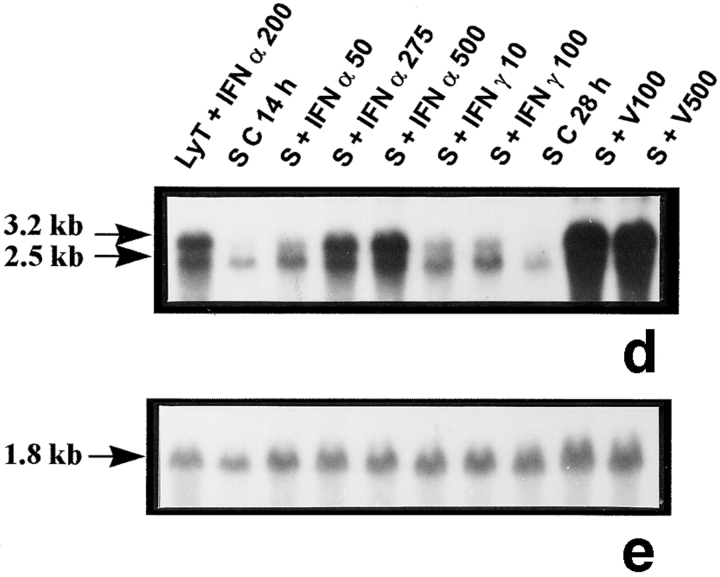
Figure 1.
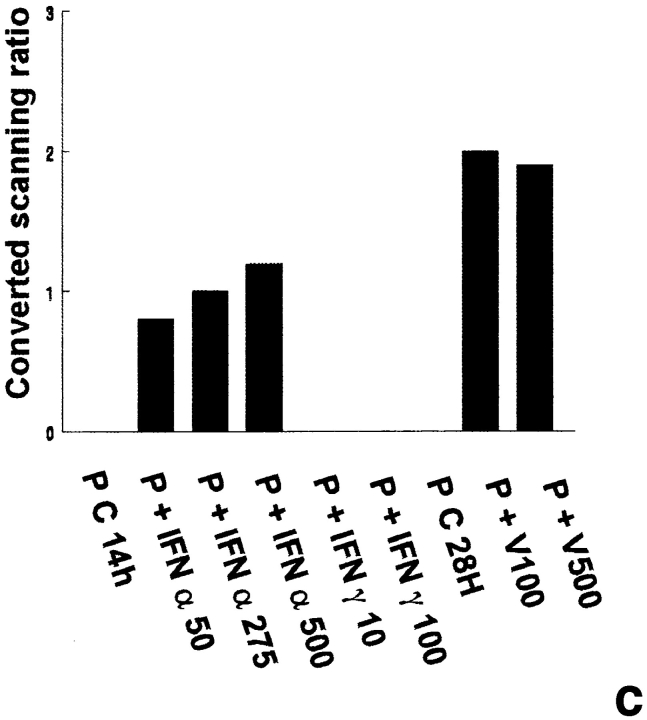
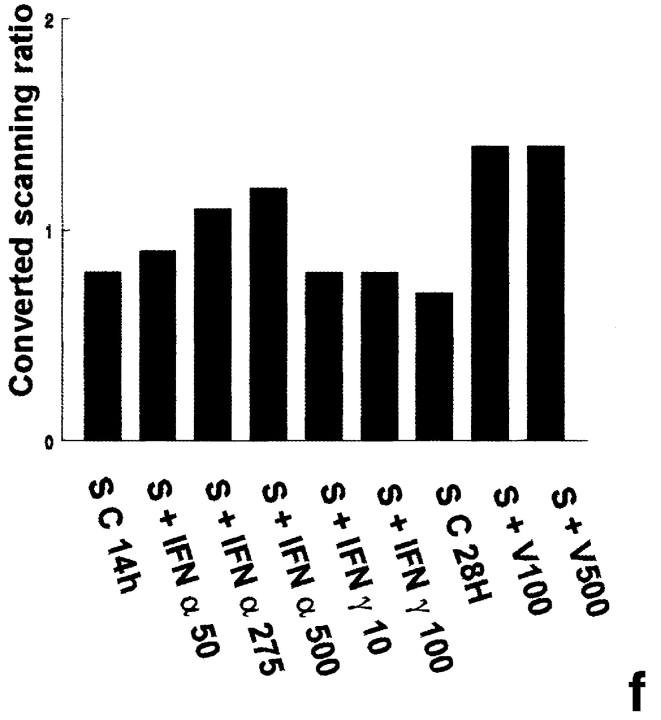
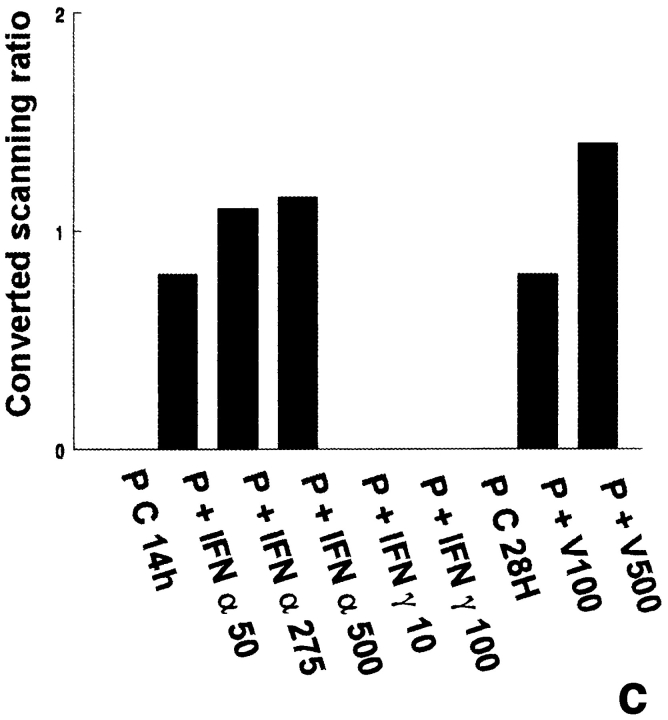
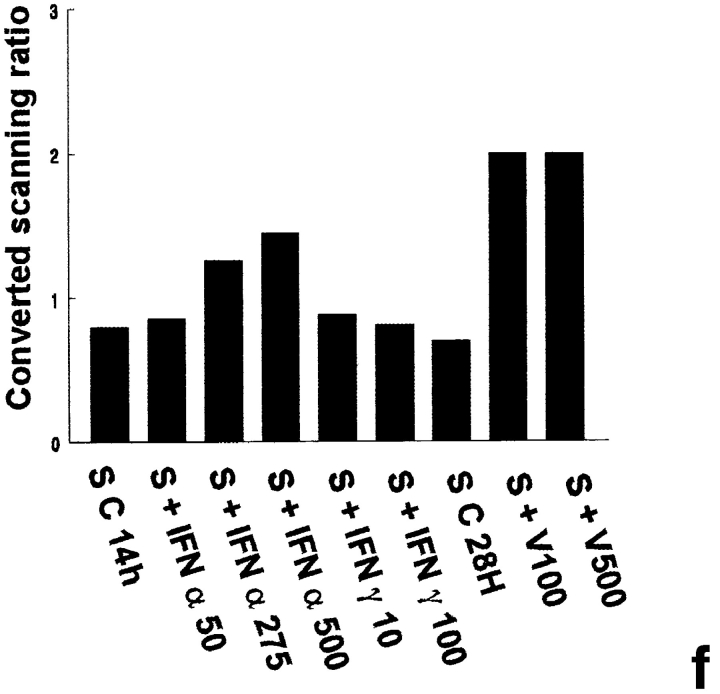
2′5′A synthetase mRNA expression in peritubular and Sertoli cells. 2′5′A synthetase mRNA expression was analyzed by Northern blot in peritubular cells (a, P) or Sertoli cells (d, S), cultured in the presence or in the absence (C) of IFN α (50, 275, and 500 U/ml) or IFN γ (10 and 100 U/ml) for 14 h, or of Sendai virus (V100 and V500 U/ml) for 28 h, using the 32P-labeled mouse 2′5′ A synthetase L3 probe. Hybridization of the blots with the actin probe is shown (b and e). The positive controls are represented by rat lymphocytes stimulated with 200 U/ml of IFN α (LyT + IFN α 200). mRNA signals were quantified by scanning densitometry and corrected relative to actin signal for both peritubular cells (c) and Sertoli cells (f). Blots shown are representative of three totally independent culture and Northern blot experiments.
Table I.
2′5′ A Synthetase (2′5′ AS), PKR, and Mx mRNAs and Proteins in Peritubular, Sertoli, and Germ Cells (Pachytene Spermatocytes and Early Spermatids) Exposed or Not to IFN α, IFN γ, or the Sendai Virus.
| Control | +IFN α | +IFN γ | + Sendai virus | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2′5′ AS | PKR | Mx | 2′5′ AS | PKR | Mx | 2′5′ AS | PKR | Mx | 2′5′ AS | PKR | Mx | |||||||||||||
| Peritubular cells | − | + | − | +++ | ++ | +++ | − | ++ | − | +++ | RNA +++ | +++ | ||||||||||||
| + Protein | ||||||||||||||||||||||||
| Sertoli cells | + | + | + | ++ | +++ | +++ | − | ++ | ++ | +++ | RNA +++ | +++ | ||||||||||||
| + Protein | ||||||||||||||||||||||||
| Germ cells | − | − | − | − | − | − | − | − | − | − | − | − | ||||||||||||
−, Absent (control) or not stimulated. +, Present (control) or slightly stimulated. ++, Markedly stimulated. +++, Dramatically stimulated.
In peritubular and Sertoli cells, IFN α was able to induce 2′5′ A synthetase mRNA expression in a dose-dependent manner, whereas IFN γ had no effect. After both IFN and Sendai virus stimulation, the main transcript evidenced was the 1.8 kb, the second strongest represented being the 2.2 kb (Fig. 1, a and d).
2′5′ A Synthetase Assay.
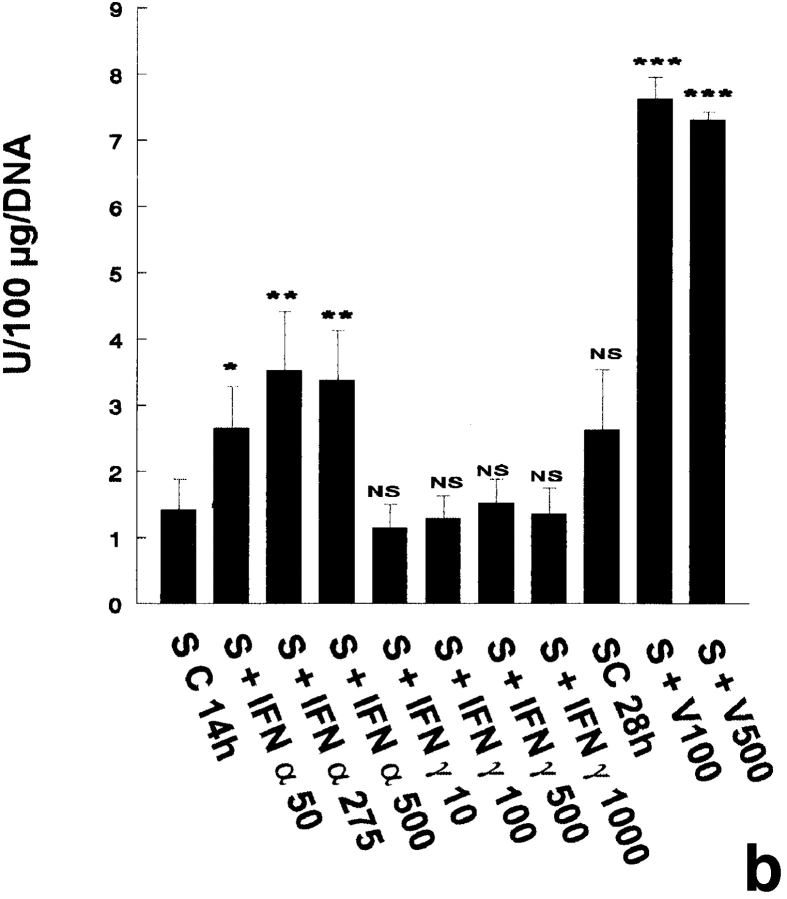
In the three independent experiments performed, no 2′5′ A synthetase activity was detected in pachytene spermatocytes and early spermatids, even when the cells were cultured with IFN α, IFN γ, or with the Sendai virus (Table I). Peritubular cells were found to express 2′5′ A synthetase activity only when cultured with IFN α or with the Sendai virus, while IFN γ had no effect on this production (Fig. 2 a). In contrast to peritubular cells, basal Sertoli cells already expressed high levels of 2′5′ A synthetase (Fig. 2 b), these levels being even higher than those produced by the IFN α–stimulated lymphocytes used as positive controls (data not shown). These basal concentrations were markedly stimulated by IFN α, but not by IFN γ, and dramatically enhanced (about fivefold) after viral stimulation (Fig. 2 b).
Figure 2.
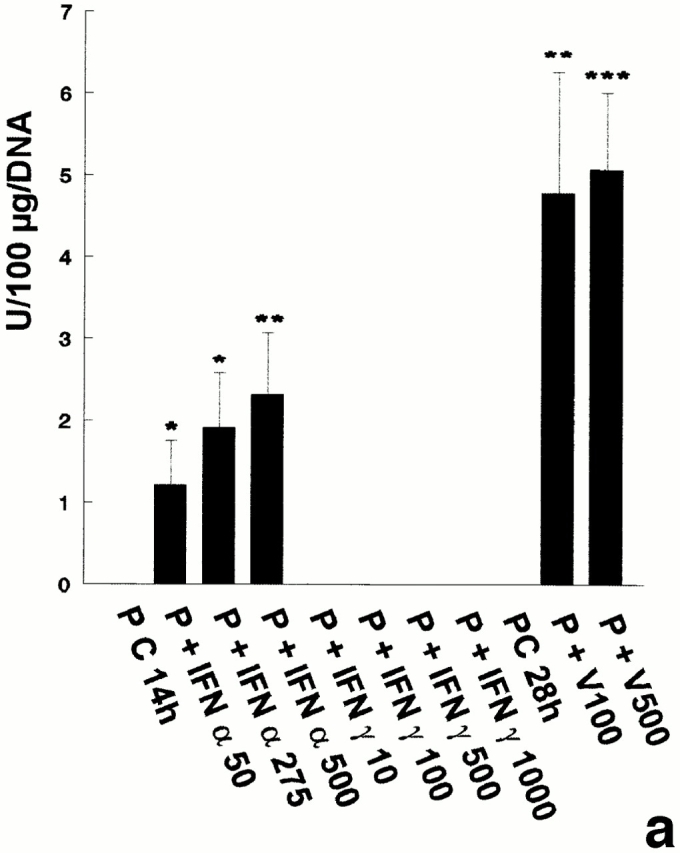
2′5′A synthetase activity in peritubular cell and Sertoli cell extracts. 2′5′A synthetase enzymatic activity was determined in the different peritubular cell (a, P) and Sertoli cell (b, S) extracts as described in Materials and Methods. Cells were stimulated or not (C) with IFN α (50, 275, and 500 U/ml) or IFN γ (10, 100, 500, and 1,000 U/ml) for 14 h, or with Sendai virus (V100 and V500 U/ml) for 28 h. Each activity value is the mean of at least three totally independent experiments. Means between stimulated and control cells were compared by Student's t test: NS, P > 0.05; *, P < 0.02; **, P = 0.001; ***, P < 0.001.
PKR Expression
Northern Blot Experiments.
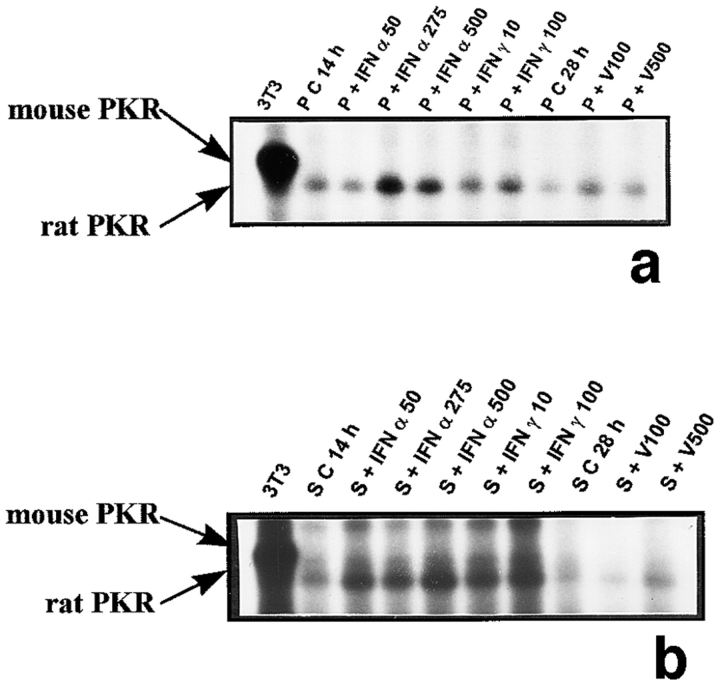
The positive control used to study PKR mRNA expression (rat lymphocytes stimulated with IFN α) revealed a single band at 4.4 kb (Fig. 3, a and d), as previously described in different rat tissues (Mellor et al., 1994). The PKR transcript was found to be very weakly expressed in basal peritubular cells but markedly increased after exposure to the Sendai virus (Fig. 3, a and c). While clearly detected in basal Sertoli cells, PKR transcript was dramatically stimulated by the virus (Fig. 3, d and f). In contrast, PKR mRNA was neither present in pachytene spermatocytes nor in early spermatids, whether the cells were exposed to the virus or not (Table I). Exposure of both peritubular and Sertoli cells to IFN α or γ resulted in a marked stimulation of PKR mRNA (Fig. 3, a and c, d and f, respectively).
Figure 3.
PKR mRNA expression in peritubular and Sertoli cells. PKR mRNA expression was analyzed by Northern blot in peritubular (a, P) and Sertoli cells (d, S) cultured in the presence or in the absence (C) of IFN α (50, 275, and 500 U/ml) or IFN γ (10 and 100 U/ml) for 14 h, or Sendai virus (V100 and V500 U/ml) for 28 h. Hybridization of the blot with the actin probe is shown (b and e). The positive control is represented by rat lymphocytes stimulated with 200 U/ml of IFN α (LyT + IFN α 200). mRNA signals were quantified by scanning densitometry and corrected relative to actin signals for both peritubular (c) and Sertoli (f) cells. Blots shown are representative of three totally independent culture and Northern blot experiments.
Protein Kinase Assay.
The rat PKR cDNA sequence has been obtained recently, and it was deduced that it encodes a protein of 513 amino acids (Mellor et al., 1994), with a molecular mass of ∼58 kD (The sequence data are available from Genbank/DDBJ/EMBL under accession number 1085566), whereas the mouse PKR is a 65-kD protein (Laurent et al., 1985). The autoradiography performed here revealed a peritubular and Sertoli cell protein migrating around 60 kD (Fig. 4, a and b) stimulated by IFN α and γ. Exposure of peritubular cells and Sertoli cells to the Sendai virus had no or only moderate stimulatory effects on PKR activity in vitro (Fig. 4, a and b).
Figure 4.
PKR activity tested in vitro on peritubular and Sertoli cell extracts. In vitro phosphorylation of PKR was performed after partial purification of the protein on poly (rI)–poly (rC) agarose, as described in Materials and Methods. Products were then analyzed on a 7.5% polyacrylamide gel (a, peritubular cells, P; b, Sertoli cells, S). Cells were stimulated or not (C) with IFN α (50, 275, and 500 U/ml) or IFN γ (10 and 100 U/ml) for 14 h, or with Sendai virus (V100 and V500 U/ml) for 28 h. The positive control is represented by murine 3T3 cells (3T3), and the autoradiography shown is representative of three totally independent culture and Northern blot experiments.
Intracellular Localization of Rat PKR.
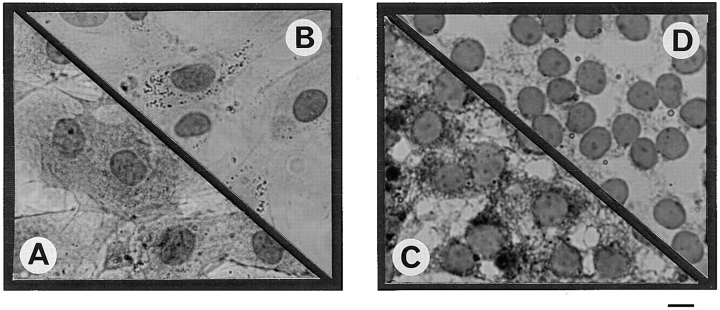
When a polyclonal rabbit antibody against mouse PKR was used, strong specific labeling was observed in both peritubular and Sertoli cell cytoplasm (Fig. 5, compare A and C with B and D, respectively). There was no difference between control cells and cells exposed to Sendai virus (data not shown).
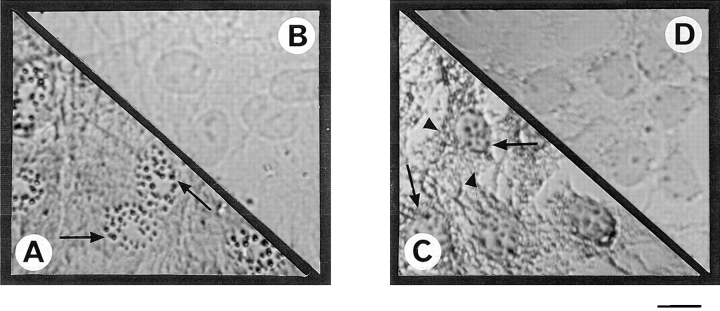
Figure 5.
Immunolocalization of PKR in peritubular and Sertoli cells. Cells were fixed after culture and permeabilized as described in Materials and Methods. Immunolocalization of PKR was performed using a rabbit polyclonal antibody against murine PKR and revealed using an avidin–biotin peroxydase complex amplification combination. Strong cytoplasmic staining was observed in both control peritubular cells (A) and Sertoli cells (C). Negative control for peritubular (B) and Sertoli cells (D) were performed using rabbit IgG at the dilution used for the PKR antibody. Bar, 15 μm.
Mx Expression
Northern Blot Experiments.
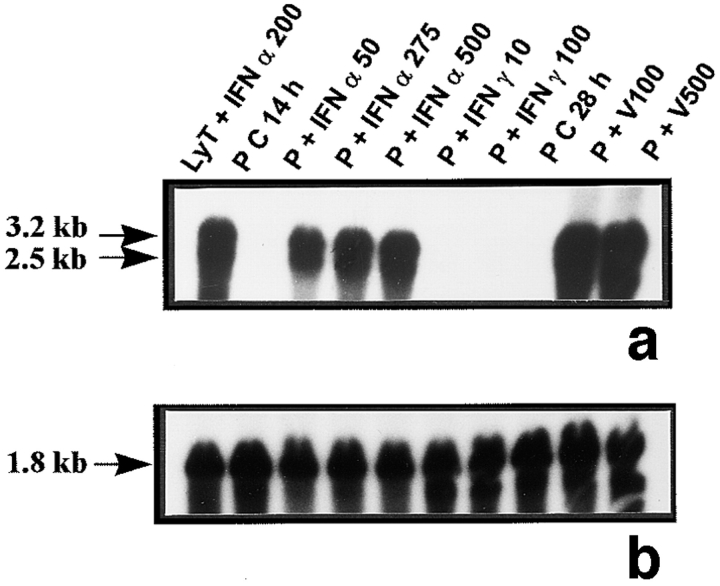
The rat lymphocytes used as positive controls revealed the two bands of 3.2 and 2.5 kb (Fig. 6, a and d) previously described in the rat and corresponding to Mx1 and Mx2 and/or Mx3 mRNAs, respectively (Meier et al., 1988). The 2.5-kb transcript was weakly expressed in untreated Sertoli cells (Fig. 6 d) but not in peritubular cells (Fig. 6 a). Exposure of both peritubular and Sertoli cells to IFN α or to Sendai virus resulted in a dramatic stimulation of both transcripts, while IFN γ had no effect on peritubular cells but induced Mx1 expression in Sertoli cells (Fig. 6, a and c, d and f, respectively). No Mx transcript was ever seen in germ cells (Table I).
Figure 6.
Mx mRNA expression in peritubular and Sertoli cells. Mx mRNA expression was analyzed by Northern blot in peritubular cells (a, P) and in Sertoli cells (d, S) cultured in the presence or in the absence of IFN α (50, 275, and 500 U/ml) or IFN γ (10 and 100 U/ml) for 14 h, or Sendai virus (V100 and V500 U/ml) for 28 h. Hybridization of the blot with the actin probe is shown (b and e). Blots shown are representative of three totally independent culture and Northern blot experiments. mRNA signals were quantified by scanning densitometry and corrected relative to actin signals for both peritubular (c) and Sertoli cells (f).
To differentiate between each Mx mRNA, we used oligonucleotides specific for rat Mx1, Mx2, and Mx3 mRNA as probes. In peritubular cells, a stronger stimulation by the virus was observed for Mx1, when compared to Mx2 and Mx3 mRNA (data not shown). In contrast to peritubular cells, Sertoli cells exposed to IFN γ presented increased Mx transcripts. The three Sertoli cell Mx mRNAs were also stimulated by IFN α and the virus (data not shown).
Immunoprecipitation of Mx Proteins.
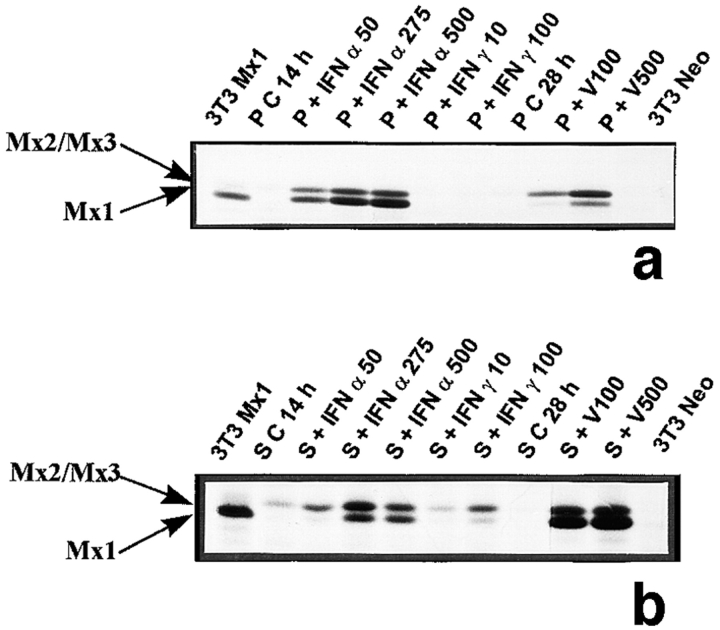
A strong band corresponding to rat Mx1 protein, which is known to migrate at ∼74 kD (Meier et al., 1990), was observed in 3T3 Mx1 cells used as positive controls (Fig. 7, a and b). Whereas in basal peritubular cells Mx proteins were not detected (Fig. 7 a), in control Sertoli cells a single band corresponding to Mx2 and/or Mx3 was present (Fig. 7 b). The relative distribution of Mx2/Mx3 and Mx1 proteins in peritubular cells after IFN α or viral stimulation was the opposite to that seen in IFN α- or virus-treated Sertoli cells. (Fig. 7 a). With both cell types, an increase in the intensity of the bands corresponding to Mx proteins was seen between 50 and 250–500 U/ml of IFN α (Fig. 7, a and b). In contrast to the results obtained with IFN α, the Mx2/Mx3 band became more abundant than that of Mx1 after exposure of peritubular cells to the Sendai virus (Fig. 7 a). Again, the reverse situation was seen in Sendai virus–exposed Sertoli cells (Fig. 7 b). Another striking difference between peritubular and Sertoli cells is that, whereas IFN γ had no effect on the expression of Mx in peritubular cells (Fig. 7 a), this cytokine induced a stimulation of both Mx1 and Mx2/Mx3 in Sertoli cells, the latter proteins being more abundant (Fig. 7 b).
Figure 7.
Expression of Mx proteins in peritubular and Sertoli cells. Mx proteins were detected by immunoprecipitation. Cells were stimulated or not (C) with IFN α (50, 275, and 500 U/ml) or IFN γ (10 and 100 U/ml) for 14 h, or with Sendai virus (V100 and V500 U/ml) for 28 h. 35S-labeled extracts were reacted with rabbit polyclonal antibody against rat/mouse Mx proteins, and products were analyzed on 7.5% polyacrylamide gel (a, peritubular cells, P; b, Sertoli cells, S). The positive and negative controls are represented by 3T3 cells transfected with the rat Mx1cDNA (3T3Mx1) and by 3T3 cells transfected with a plasmid lacking the MX1cDNA (3T3Neo), respectively. Blots shown are representative of three totally independent culture and immunoprecipitation experiments.
Intracellular Localization of Rat Mx Proteins.
The same results were observed with the two different antibodies used: while no staining was observed in basal peritubular cells (Fig. 8 B), a strong specific staining was observed in both the nucleus (characteristic granules) and the cytoplasm of Sendai virus–exposed peritubular cells (Fig. 8 A). Similarly, in basal Sertoli cells, no staining was observed without stimulus (Fig. 8 D), whereas characteristic granules appeared in the virus-exposed Sertoli cell nucleus and in the cytoplasm (Fig. 8 C). That no immunostaining was observed in basal Sertoli cells most probably results from the relatively low sensitivity of the immunocytochemistry technique.
Figure 8.
Immunolocalization of Mx proteins in peritubular and in Sertoli cells. Cells were fixed after culture and permeabilized as described in Materials and Methods. Immunolocalization of Mx proteins was performed using a mouse monoclonal antibody (2C12) against rat/mouse Mx proteins and revealed using an avidin–biotin peroxidase complex amplification combination. No staining was observed in either control peritubular cells (B) or Sertoli cells (D). In contrast, peritubular cells stimulated with 500 U/ml of Sendai virus (A) showed strong granular staining in the nucleus (arrows) and a more diffuse staining in the cytoplasm, whereas Sertoli cells stimulated with the same concentration of virus (C) showed a granular staining in the nucleus (arrows) and a diffuse and granular staining in the cytoplasm (arrowheads). Bar, 15 μm.
Discussion
Entry of viruses within the testis can occur either by the interstitial blood and lymphatic vessels or through the albugina, which envelops the testicular parenchyma (Mikuz and Damjanov, 1982). Mumps, HIV, hepatitis B and C viruses, and Herpes Simplex virus have been found in the interstitium and/or the seminiferous tubules in men (Mikuz and Damjanov, 1982; Csata and Kulcsar, 1991; Lang, 1993; Liu, 1994 Nuovo et al., 1994). Furthermore, some of these viruses, like HIV and hepatitis B virus, are also present in semen and known to be transmissible via this biological vector (Xu, 1992; Baccetti et al., 1994). To understand how the cells of the seminiferous epithelium are able to react to a viral infection is, therefore, of prime importance for the understanding of the circumstances that lead to a seminiferous viral infection, and possibly to sperm and semen contaminations.
Over the last decades, important progress has been made in the elucidation of the major cellular antiviral mechanisms in many tissues. In this context, IFN production represents a key event in the cell response to a viral infection. Therefore, as a first step in the study of the potential testicular anti-viral system, we recently studied IFN expression within the seminiferous tubules (Dejucq et al., 1995).
Our major goal here was to investigate for the presence of the three best known IFN-induced antiviral proteins, namely 2′5′ A synthetase, PKR, and Mx, within the seminiferous tubules. As a first approach, we initially thought of using immunohistochemical techniques to establish the cellular topography of expression of these proteins. However, there were no rat/mouse 2′5′ A synthetase antibodies available, and preliminary experiments performed with PKR and Mx antibodies did not succeed, probably due to the low basal levels of these proteins. This, and the fact that serious side effects due to the administration of IFNs or Sendai virus to the rat could be observed, led us to study the cellular distribution of 2′5′ A synthetase, PKR, and Mx and their regulation using different isolated cell types prepared from the rat seminiferous tubules.
The first IFN-induced antiviral protein studied was the 2′5′ A synthetase. This enzyme requires double-stranded RNA (ds RNA), the usual replicating intermediate of RNA viruses, as activator (Sen and Lengyel, 1992). 2′5′ A synthetase polymerizes ATP into a series of 2′5′ oligoadenylates that, in turn, activate a latent ubiquitous endoribonuclease, RNase L. Once activated, RNase L degrades viral and cellular single-stranded RNAs (Williams and Fish, 1985). There are multiple isoenzymes of 2′5′ A synthetase in humans and mice, all of which are induced by both type I and type II IFNs. In mouse cells, several cDNAs for small (∼40 kD) mouse 2′5′ A synthetases have been cloned (Coccia et al., 1990; Ghosh et al., 1991). In IFN-treated mouse L cells, Rutherford et al. (1991) have identified three related transcripts. Similarly, in the present experiments, three transcripts of 1.8, 2.2, and 4 kb were detected in IFN-treated L929 cells as well as in rat Sertoli and peritubular cells, but never in germ cells. The most represented mRNA in the seminiferous tubule somatic cells appears to be by far the 1.8 kb that, according to Coccia et al. (1990) corresponds to a 2′5′ A synthetase of low molecular weight. Our results show that 2′5′ A synthetase is expressed in basal Sertoli cells and increased by IFN α and Sendai virus, whereas IFN γ had no effect. The same pattern was found in peritubular cells, except that no constitutive expression was observed (Table I).
The second IFN-induced ds RNA-dependent enzyme that we studied was PKR. The autophosphorylation of PKR, in the presence of ds RNA (or others polyanions) confers to this protein the ability to phosphorylate other substrates, most prominently the α subunit of the eukaryotic translation initiation factor 2 (eIF2α; Galabru and Hovanessian, 1987). Phosphorylation of eIF2α in turn inhibits viral protein production and, thereby, greatly reduces production of new virus particles (O'Malley et al., 1986). PKR protein and mRNA were never detected in meiotic and post-meiotic germ cells. In contrast, Sertoli and peritubular cells constitutively express PKR, and this expression was stimulated by IFNs (Table I). Sendai virus exposure highly increased PKR mRNA levels but seemed to have no effect on the protein activity, as detected by our in vitro autophosphorylation experiments. There are at least two possible explanations for the latter results, that are not exclusive: the Sendai virus could possibly catalyze the activation of PKR within the cells, which would normally lead to an in vivo autophosphorylation of this protein that cannot occur or be detected in our in vitro system; it is also possible that the Sendai virus can inhibit PKR activation, like other viruses previously described (Polyack et al., 1996). To our knowledge nothing is presently known about the abilities of Sendai virus to inhibit PKR.
The last proteins studied here were of the Mx family. The IFN-regulated Mx gene has been shown to mediate selective resistance to Influenza virus in mice (Staeheli and Haller, 1987). Meier et al. (1990) have described three Mx proteins, deriving from three distinct genes, in IFN α/β-treated rat embryo fibroblasts. Mx1 is a nuclear protein that inhibits both Influenza virus and VSV, whereas Mx2 and Mx3 are cytoplasmic proteins, inhibiting VSV (Mx2) or devoid of antiviral activity (Mx3). Our study reveals that Sertoli cells constitutively express relatively low levels of Mx2 and Mx3, but no Mx1. The latter protein appeared only after IFN α, IFN γ or Sendai virus stimulation, while Mx2 and Mx3 mRNAs and proteins were increased by these three stimuli (Table I). To our knowledge, this is the first time that Mx proteins are found to be constitutively expressed in a cell type and to display differential patterns of expression between themselves, after exposure to various stimuli. Moreover, IFN γ, which usually has no effect on Mx protein regulation, is able in Sertoli cells to induce Mx1 expression and to stimulate Mx2 and Mx3 expression. In contrast to what was observed in Sertoli cells, no Mx protein or mRNA was detected in basal peritubular cells or after IFN γ treatment. However, Mx1, Mx2, and Mx3 were also found in peritubular cells after IFN α or Sendai virus exposure but displayed different patterns of expression to those seen in Sertoli cells. As for 2′5′ A synthetase and PKR, neither Mx mRNA nor proteins were detected in germ cells.
Importantly, this study reveals that the Sendai virus is able to induce a very strong stimulation of 2′5′ A synthetase, PKR, and Mx mRNA expression, as well as 2′5′ A synthetase and Mx protein level in peritubular and Sertoli cells. To our knowledge, the only previous publication dealing with a potential IFN-induced antiviral activity in testicular cells was that of Bosworth and MacLachlan (1990), who studied IFNα1-induced 2′5′ A synthetase activity by swine testicular cells in culture. However, the results obtained by these authors cannot really be interpreted as the nature of the cells and of the cell line used was not indicated. In the present study, the incubation time of the cells with the virus was long enough (28 h) to allow the synthesis of IFNs by the virus-stimulated cells and to allow, in turn, the IFNs produced to induce the synthesis of the proteins of interest. At present, we do not know why more pronounced effects of the Sendai virus were always observed on IFN-induced protein synthesis, comparatively to the effects of rat recombinant IFN α. Indeed, it can be excluded that the more pronounced effects of the Sendai virus result from the induction of higher concentrations of IFN α in peritubular and Sertoli cells than the ones added to the media, as up to 1,000 U/ml of IFN α always displayed negative results (data not shown). Furthermore, the highest concentrations of recombinant IFN α used here were chosen to match the concentrations of type I IFN produced by peritubular and Sertoli cells, in response to high concentrations of Sendai virus (Dejucq et al., 1995). However, it cannot be excluded that the Sendai virus induced–IFNs represent a mixture of several type I IFNs that is more efficient than the rat recombinant IFN α protein used alone. Another explanation for this difference is that the virus may exert a direct effect on protein synthesis, an effect not mediated by IFN synthesis, as previously shown for Mx proteins and for 2′5′ A synthetase (Hug et al., 1988; Goetschy et al., 1989; Mémet et al., 1991). In favor of this last hypothesis is the fact that the pattern of expression of peritubular and Sertoli cells' Mx proteins after viral stimulation is the opposite of that observed after IFN α stimulation.
A major fact emerges from the present study: the seminiferous tubules are very well equipped to react to a viral attack, and this potential antiviral defense system is assumed solely by peritubular and Sertoli cells, since pachytene spermatocytes and early spermatids lack the three major IFN-induced antiviral proteins studied. These latter germ cell types were also previously shown to be unable or only marginally able to produce type I IFN in response to a Sendai virus exposure (Dejucq et al., 1995). Of note are the differences observed in the patterns of protein expression between Sertoli and peritubular cells: only Sertoli cells constitutively express 2′5′ A synthetase, PKR, and Mx2/ Mx3 proteins, and IFN γ action was restricted to PKR in peritubular cells (Table I). We therefore hypothetize that these differential and complementary patterns of expression between the cells bordering the tubules and Sertoli cells generate a much higher efficiency in the tubule antiviral defense system than if the pattern of expression were identical in both cell types. Most interestingly, the cellular support of this potential antiviral defense system is strictly coincident to the tubular blood testis barrier, which, in rodents, is also assumed by peritubular and Sertoli cells (Ploën and Setchell, 1992). Therefore, from the data presented here, it appears that the concept of the existence of a specific intratubular microenvironment, so far applied to the nutrition, regulation, and immune protection of the meiotic and post-meiotic germ cells, can also be extended to the germ cell antiviral defense. As in the tubules, IFN γ was previously found to be produced by early spermatids (Dejucq et al., 1995), and since this type II IFN is able to stimulate PKR and Mx expression in Sertoli cells (Table I), it could also be that the somatic tubular defense system is complemented by a paracrine loop of regulation whereby assaulted haploid cells may control aspects of their own defense, via the stimulation of Sertoli cell antiviral proteins. It remains to be elucidated in which infectious states this overall potential antiviral defense system is operational in situ, as it is known that some viruses are able to overcome this system and to alter spermatogenesis and thereby possibly contaminate spermatozoa and semen.
Acknowledgments
We thank Anne-Marie Touzalin for technical assistance in preparing germ cells, Marie-Odile Liénard, Laurence Fornari, and Christiane Legouëvec for their help with the preparation of the manuscript and Louis Communier for the photography work. We are also very grateful to Pr. Haller for advice in Mx search.
This work was supported by Institut National de la Santé et de la Recherche Medical, the Ministère de l'Education Nationale, de L'Enseignement Supérieur et de la Recherche, and Région Bretagne. N. Dejucq was a recipient of a Conseil Régional de Bretagne fellowship.
Abbreviations used in this paper
- ds
double stranded
- HIV
human immunodeficiency virus
- IFN
interferon
- PKR
RNA-activated protein kinase
Footnotes
Address all correspondence to Bernard Jégou, GERM-INSERM U 435, Université de Rennes I, Campus de Beaulieu, 35 042 Rennes Cedex, Bretagne, France. Tel.: (33) 299-28-69-11. Fax: (33) 299-28-16-13. E-mail: bernard.jegou@rennes,inserm.fr
References
- Alexander NJ. Sexual transmission of human immunodeficiency virus: virus entry into the male and female genital tract. Fertil Steril. 1990;54:1–18. doi: 10.1016/s0015-0282(16)53628-0. [DOI] [PubMed] [Google Scholar]
- Baccetti B, Benedetto A, Burrini AG, Collodel G, Ceccarini EC, Crisa N, DiCaro A, Estenoz M, Garbuglia AR, Massacesi A, et al. HIV-particles in spermatozoa of patients with AIDS and their transfer into the oocyte. J Cell Biol. 1994;127:903–914. doi: 10.1083/jcb.127.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosworth BT, MacLachlan NJ. Evaluation of age-related effects on the antiviral activity of interferon and induction of 2-5A synthetases in testicular cell cultures derived from swine of various ages. Am J Vet Res. 1990;51:723–725. [PubMed] [Google Scholar]
- Chapin RE, Phelps JL, Miller BE, Gray TJB. Alkaline phosphatase histochemistry discriminates peritubular cells in primary rat testicular cell culture. J Androl. 1987;8:155–161. doi: 10.1002/j.1939-4640.1987.tb02427.x. [DOI] [PubMed] [Google Scholar]
- Chousterman S, Chelbi-Alix MK, Thang MN. 2′5′-oligoadenylate synthetase expression is induced in response to heat shock. J Biol Chem. 1987;262:4806–4811. [PubMed] [Google Scholar]
- Coccia EM, Romeo G, Nissim A, Marziali G, Albertini R, Affabris E, Battistini A, Fiorucci G, Orsatti R, Rossi GB, et al. A full-length murine 2-5A synthetase cDNA transfected in NIH-3T3 cells impairs EMCV but not VSV replication. Virology. 1990;179:228–233. doi: 10.1016/0042-6822(90)90292-y. [DOI] [PubMed] [Google Scholar]
- Csata S, Kulcsar G. Virus host studies in human seminal and mouse testicular cells. Acta Chir Hung. 1991;321:83–90. [PubMed] [Google Scholar]
- Dejucq N, Dugast I, Ruffault A, Van der Meide PH, Jégou B. Interferon α and γ expression in the rat testis. Endocrinolgogy. 1995;136:4925–4931. doi: 10.1210/endo.136.11.7588226. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to hogh specific. Anal Biochem. 1993;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feng GS, Chong K, Kumar A, Williams BRG. Identification of double stranded RNA-binding domains in the interferon-induced double-stranded RNA-activated p68 kinase. Proc Natl Acad Sci USA. 1992;89:5447–5451. doi: 10.1073/pnas.89.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galabru J, Hovanessian AG. Autophosphorylation of the protein kinase dependent on double-stranded RNA. J Biol Chem. 1987;262:15538–15544. [PubMed] [Google Scholar]
- Galdieri MK, Ziparo E, Palombi F, Russo MA, Stefanini M. Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J Androl. 1981;5:249–254. [Google Scholar]
- Ghosh SK, Kusari J, Bandyopadhyay SK, Samanta H, Kumar R, Sen GC. Cloning, sequencing, and expression of two murine 2′-5′-oligodenylate synthetases. J Biol Chem. 1991;266:15293–15299. [PubMed] [Google Scholar]
- Goetschy JF, Zeller H, Content J, Horisberger MA. Regulation of the interferon-inducible IFI-78 K gene, the human equivalent of the murine Mx gene, by interferons, double-stranded RNA, certain cytokines, and viruses. J Virol. 1989;63:2616–2622. doi: 10.1128/jvi.63.6.2616-2622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekman ACP, Trapman J, Mulder AH, Van Gaalen JLM, Zwarthoff EC. Interferon expression in the testes of transgenic mice leads to sterility. J Biol Chem. 1988;263:12151–12155. [PubMed] [Google Scholar]
- Hug H, Costas M, Staeheli P, Aebi M, Weissmann C. Organization of the murine Mx gene and characterization of its interferon- and virus-inducible promoter. Mol Cell Biol. 1988;8:3065–3079. doi: 10.1128/mcb.8.8.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y, Asano M, Nishimune Y, Kawade Y. Male sterility of transgenic mice carrying exogenous mouse interferon-β gene under the control of the metallothionein enhancer - promoter. EMBO (Eur Mol Biol Organ) J. 1988;7:3757–3762. doi: 10.1002/j.1460-2075.1988.tb03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jégou B. The Sertoli-germ cell communication network in mammals. Int Rev Cytol. 1993;147:25–96. [PubMed] [Google Scholar]
- Jégou B. Les hommes deviennent-ils moins fertiles? . La Recherche. 1996;288:60–65. [Google Scholar]
- Lang ZW. Distribution of hepatitis B virus in testicle tissue in patients with hepatitis B infection. Chung Hua I Hsueh Tsa Chih. 1993;73:329–331. [PubMed] [Google Scholar]
- Laurent AG, Krust B, Galabru J, Svab J, Hovanessian AG. Monoclonal antibodies to an interferon-induced Mr 68,000 protein and their use for the detection of double-stranded RNA-dependent protein kinase in human cells. Proc Natl Acad Sci USA. 1985;82:4341–4345. doi: 10.1073/pnas.82.13.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FH, Tian GS, Fu XX. Detection of plus and minus strand hepatitis C virus RNA in peripheral blood mononuclear cells and spermatid. Chung Hua I Hsueh Tsa Chih. 1994;74:284–286. [PubMed] [Google Scholar]
- Meier E, Fah J, Grob MS, End R, Staeheli P, Haller O. A family of interferon-induced Mx-related mRNAs encodes cytoplasmic and nuclear proteins in rat cells. J Virol. 1988;62:2386–2393. doi: 10.1128/jvi.62.7.2386-2393.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier E, Kunz G, Haller O, Arnheiter H. Activity of rat Mx proteins against a rhabdovirus. J Virol. 1990;64:6263–6269. doi: 10.1128/jvi.64.12.6263-6269.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H, Flowers KM, Scot RK, Jefferson LS. Cloning and characterization of a cDNA encoding rat PKR, the double-stranded RNA-dependent eukaryotic initiation factor-2 kinase. Biochim Biophys Acta. 1994;1219:693–696. doi: 10.1016/0167-4781(94)90229-1. [DOI] [PubMed] [Google Scholar]
- Mémet S, Besançon F, Bourgeade MF, Thang MN. Direct induction of interferon-β and interferon-α/-β inducible genes by double-stranded RNA. J Interferon Res. 1991;11:131–141. doi: 10.1089/jir.1991.11.131. [DOI] [PubMed] [Google Scholar]
- Mikuz G, Damjanov I. Inflammation of the testis, epididymis, peritesticular membranes and scrotum. Pathobiol Annu. 1982;17:101–128. [PubMed] [Google Scholar]
- Nuovo GJ, Becker J, Simsir A, Margiotta M, Khalife G, Shevchuk M. HIV-1 nucleic acids localize to the spermatogonia and their progeny. A study by polymerase chain reaction in situ hybridization. Am J Pathol. 1994;144:1142–1148. [PMC free article] [PubMed] [Google Scholar]
- O'Malley RP, Marion TM, Siekierka J, Mathews MA. A mechanism for the control of protein synthesis by adenovirus VAIRNA. Cell. 1986;44:391–400. doi: 10.1016/0092-8674(86)90460-5. [DOI] [PubMed] [Google Scholar]
- Petryshyn R, Chen JJ, London IM. Detection of activated double-stranded RNA-dependent protein kinase in 3T3-F442A cells. Proc Natl Acad Sci USA. 1988;85:1427–1431. doi: 10.1073/pnas.85.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau C, Le Magueresse B, Courtens JL, Jégou B. Study in vitro of the phagocytic function of Sertoli cells in the rat. Cell Tissue Res. 1991;264:589–598. doi: 10.1007/BF00319048. [DOI] [PubMed] [Google Scholar]
- Pineau C, Syed V, Bardin CW, Jégou B, Cheng CY. Germ cell-conditionned medium contains multiple factors that modulate the secretion of testins, clusterin, and transferrin by Sertoli cells. J Androl. 1993;14:87–98. [PubMed] [Google Scholar]
- Plöen L, Setchell P. Blood-testis barriers revisited. A homage to Lennart Nicander. Int J Androl. 1992;15:1–4. doi: 10.1111/j.1365-2605.1992.tb01108.x. [DOI] [PubMed] [Google Scholar]
- Polyack SJ, Tang N, Wambach M, Barber GN, Katze MG. The P58 cellular inhibitor complexes with the interferon-induced double stranded RNA-dependent protein kinase, PKR, to regulate its autophosphorylation and activity. J Biol Chem. 1996;271:1702–1707. doi: 10.1074/jbc.271.3.1702. [DOI] [PubMed] [Google Scholar]
- Raymondjean M, Kneip B, Schapira G. Preparation and characterization of mRNAs from rat heart muscle. Biochemistry. 1983;65:65–70. doi: 10.1016/s0300-9084(83)80030-3. [DOI] [PubMed] [Google Scholar]
- Rutherford MN, Kumar A, Nissim A, Chebath J, Williams BRG. The murine 2-5A synthase locus: three distinct transcripts from linked genes. Nucleic Acids Res. 1991;19:1917–1924. doi: 10.1093/nar/19.8.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg S, Mandelbaum M, Zalcberg M, Shainberg A. Interruption of myogenesis by transforming growth factor 1 or EGTA inhibits expression and activity of the myogenic-associated 2′-5′ oligoadenylate synthetase and PKR. Exp Cell Res. 1995;219:223–232. doi: 10.1006/excr.1995.1222. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991;183:1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- Scott LS. Mumps and male fertility. Br J Urol. 1960;32:183. doi: 10.1111/j.1464-410x.1960.tb03761.x. [DOI] [PubMed] [Google Scholar]
- Sen GC, Lengyel P. The interferon system. A bird's eye view of its biochemistry. J Biol Chem. 1992;267:5017–5020. [PubMed] [Google Scholar]
- Skinner MK, Fritz IB. Testicular peritubular cells secrete a protein under androgen control that modulates Sertoli cell functions. Proc Natl Acad Sci USA. 1985;82:114–118. doi: 10.1073/pnas.82.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P. Interferon-induced proteins and the antiviral state. Adv Virus Res. 1990;38:147–200. doi: 10.1016/s0065-3527(08)60862-3. [DOI] [PubMed] [Google Scholar]
- Staeheli P, Haller O. Interferon-induced human protein with homology to protein Mx of influenza virus-resistant mice. Mol Cell Biol. 1985;5:2150–2153. doi: 10.1128/mcb.5.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P, Haller O. Interferon induced Mx protein: a mediator of cellular resistance to influenza virus. Interferon. 1987;8:1–23. [PubMed] [Google Scholar]
- Staeheli P, Dreiding P, Haller O, Lindenmann J. Polyclonal and monoclonal antibodies to the interferon-inducible protein Mx of influenza virus-resistant mice. J Biol Chem. 1985;260:1821–1825. [PubMed] [Google Scholar]
- Staeheli P, Haller O, Boll W, Lindenmann J, Weissmann C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to Influenza virus. Cell. 1986;44:147–158. doi: 10.1016/0092-8674(86)90493-9. [DOI] [PubMed] [Google Scholar]
- Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ, Jégou B, Jensen TK, Jouannet P, Keiding N, et al. Male reproductive health and environmental chemicals with estrogenic effects. Environ Health Perspect. 1996;104:741–776. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, B.R.G., and E.N. Fish. 1985. Interferons and viruses: in vitro studies. In Interferons: Their Impact in Biology and Medicine. J. Taylor-Papadimitriou, editor. Oxford University Press, Oxford. 40–60..
- Xu X. The possible role of sperm in family HBV infection. Chung Hua Liu Hsing Ping Hsueh Tsa Chih. 1992;13:337–339. [PubMed] [Google Scholar]