Abstract
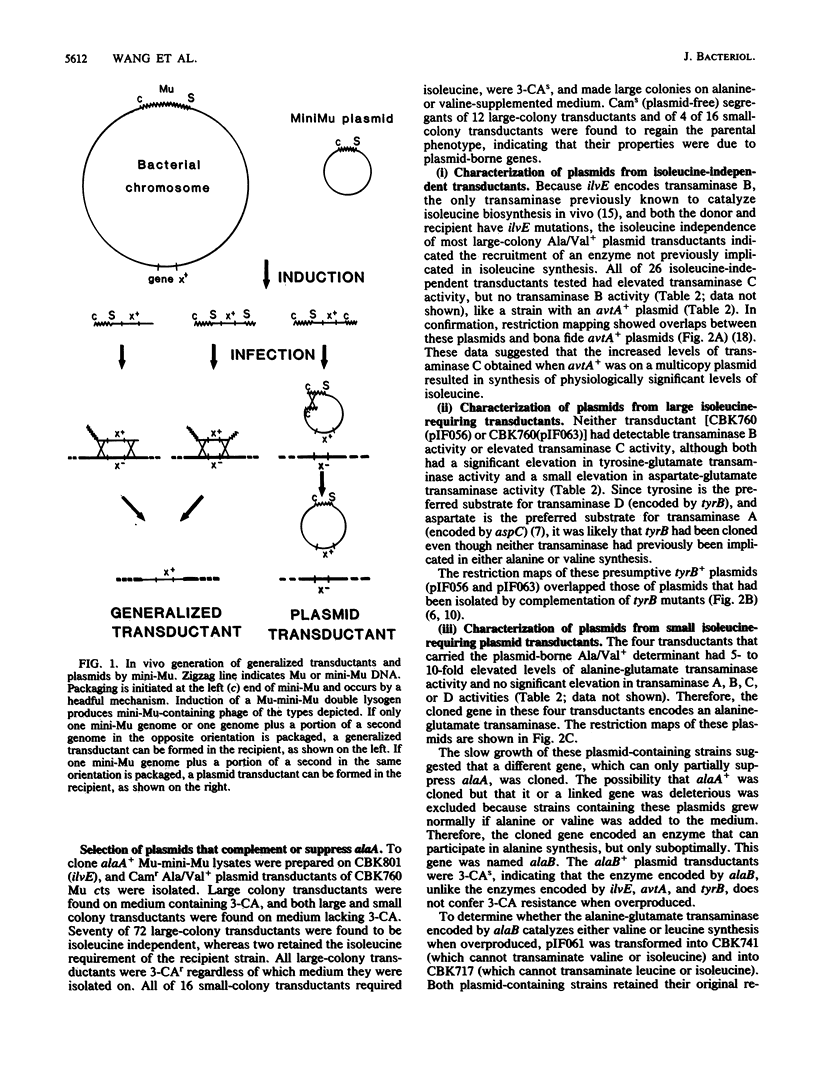
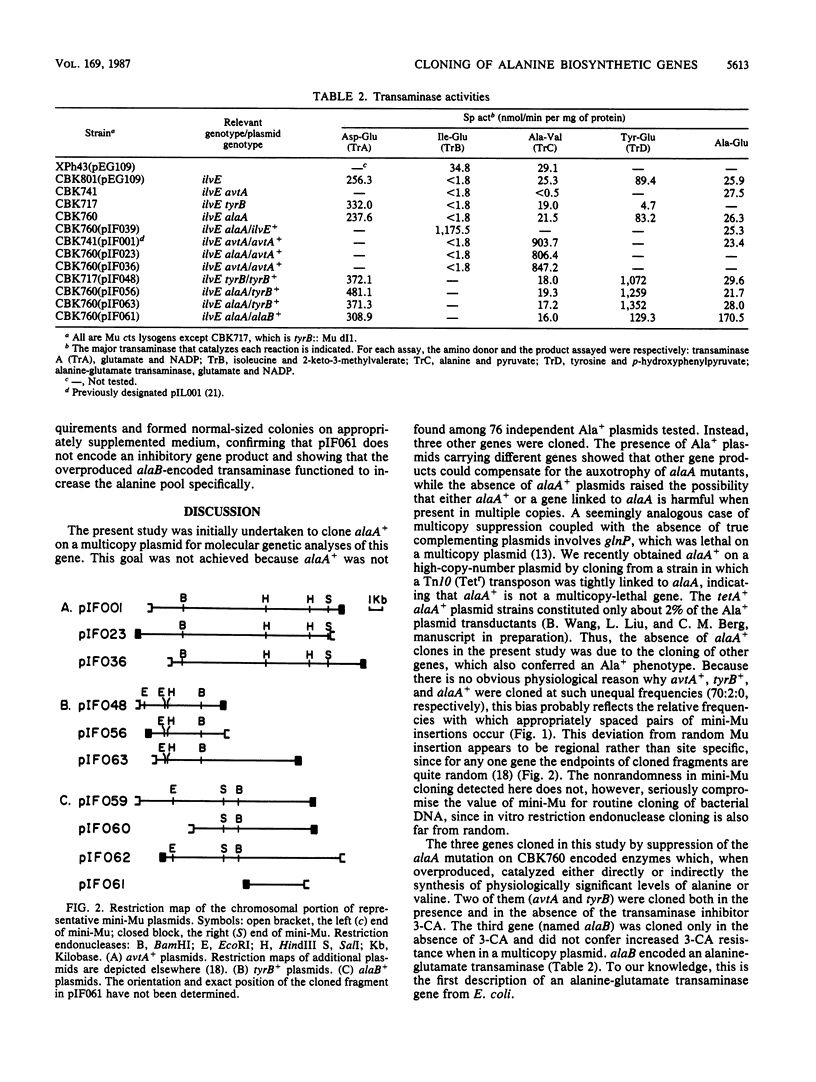
To facilitate molecular analyses of a previously uncharacterized gene involved in alanine synthesis, attempts were made to clone the wild-type allele of this gene, alaA, with a mini-Mu plasmid element used for in vivo cloning. Seventy-six independent Ala+ plasmids were isolated and characterized. Physiological, enzymological, and restriction endonuclease analyses indicated that three different genes, none of them alaA, were cloned. These genes were avtA+, which encodes the alanine-valine transaminase (transaminase C); tyrB+, which encodes the tyrosine-repressible transaminase (transaminase D); and a previously undescribed gene, called alaB, which encodes an alanine-glutamate transaminase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfin S. M., Koziell D. A. Inhibition of growth of Salmonella typhimurium and of threonine deaminase and transaminase B by beta-chloroalanine. J Bacteriol. 1971 Feb;105(2):519–522. doi: 10.1128/jb.105.2.519-522.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg C. M., Shaw K. J., Vender J., Borucka-Mankiewicz M. Physiological characterization of polar Tn5-induced isoleucine-valine auxotrophs in Escherichia coli K.12: evidence for an internal promoter in the ilvOGEDA operon. Genetics. 1979 Oct;93(2):308–319. [PMC free article] [PubMed] [Google Scholar]
- Berg C. M., Whalen W. A., Archambault L. B. Role of alanine-valine transaminase in Salmonella typhimurium and analysis of an avtA::Tn5 mutant. J Bacteriol. 1983 Sep;155(3):1009–1014. doi: 10.1128/jb.155.3.1009-1014.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman E., Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and phi80 transducing phages. J Mol Biol. 1975 Aug 5;96(2):307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- Fotheringham I. G., Dacey S. A., Taylor P. P., Smith T. J., Hunter M. G., Finlay M. E., Primrose S. B., Parker D. M., Edwards R. M. The cloning and sequence analysis of the aspC and tyrB genes from Escherichia coli K12. Comparison of the primary structures of the aspartate aminotransferase and aromatic aminotransferase of E. coli with those of the pig aspartate aminotransferase isoenzymes. Biochem J. 1986 Mar 15;234(3):593–604. doi: 10.1042/bj2340593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand D. H., Steinberg R. A. Escherichia coli mutants deficient in the aspartate and aromatic amino acid aminotransferases. J Bacteriol. 1977 Apr;130(1):429–440. doi: 10.1128/jb.130.1.429-440.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman E. A., Castilho B. A., Casadaban M. J. In vivo DNA cloning and adjacent gene fusing with a mini-Mu-lac bacteriophage containing a plasmid replicon. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1480–1483. doi: 10.1073/pnas.81.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe M. M. Prophage deletion mapping of bacteriophage Mu-1. Virology. 1973 Jul;54(1):93–101. doi: 10.1016/0042-6822(73)90118-9. [DOI] [PubMed] [Google Scholar]
- Kuramitsu S., Inoue K., Ogawa T., Ogawa H., Kagamiyama H. Aromatic amino acid aminotransferase of Escherichia coli: nucleotide sequence of the tyrB gene. Biochem Biophys Res Commun. 1985 Nov 27;133(1):134–139. doi: 10.1016/0006-291x(85)91851-0. [DOI] [PubMed] [Google Scholar]
- McGilvray D., Umbarger H. E. Regulation of transaminase C synthesis in Escherichia coli: conditional leucine auxotrophy. J Bacteriol. 1974 Nov;120(2):715–723. doi: 10.1128/jb.120.2.715-723.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohno T., Saito T., Hong J. S. Cloning and complete nucleotide sequence of the Escherichia coli glutamine permease operon (glnHPQ). Mol Gen Genet. 1986 Nov;205(2):260–269. doi: 10.1007/BF00430437. [DOI] [PubMed] [Google Scholar]
- Primerano D. A., Burns R. O. Role of acetohydroxy acid isomeroreductase in biosynthesis of pantothenic acid in Salmonella typhimurium. J Bacteriol. 1983 Jan;153(1):259–269. doi: 10.1128/jb.153.1.259-269.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDMAN D., MEISTER A. Transamination in Escherichia coli. J Biol Chem. 1953 Feb;200(2):591–604. [PubMed] [Google Scholar]
- Shaw K. J., Berg C. M., Sobol T. J. Salmonella typhimurium mutants defective in acetohydroxy acid synthases I and II. J Bacteriol. 1980 Mar;141(3):1258–1263. doi: 10.1128/jb.141.3.1258-1263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. M., Liu L., Groisman E. A., Casadaban M. J., Berg C. M. High frequency generalized transduction by miniMu plasmid phage. Genetics. 1987 Jun;116(2):201–206. doi: 10.1093/genetics/116.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. D., Liu L., Wang B. M., Berg C. M. Cloning and characterization of the Escherichia coli K-12 alanine-valine transaminase (avtA) gene. J Bacteriol. 1987 Sep;169(9):4228–4234. doi: 10.1128/jb.169.9.4228-4234.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman S. A., Walsh C. T., Botstein D. Two alanine racemase genes in Salmonella typhimurium that differ in structure and function. J Bacteriol. 1983 Mar;153(3):1439–1450. doi: 10.1128/jb.153.3.1439-1450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen W. A., Berg C. M. Analysis of an avtA::Mu d1(Ap lac) mutant: metabolic role of transaminase C. J Bacteriol. 1982 May;150(2):739–746. doi: 10.1128/jb.150.2.739-746.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen W. A., Wang M. D., Berg C. M. beta-Chloro-L-alanine inhibition of the Escherichia coli alanine-valine transaminase. J Bacteriol. 1985 Dec;164(3):1350–1352. doi: 10.1128/jb.164.3.1350-1352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


