Abstract
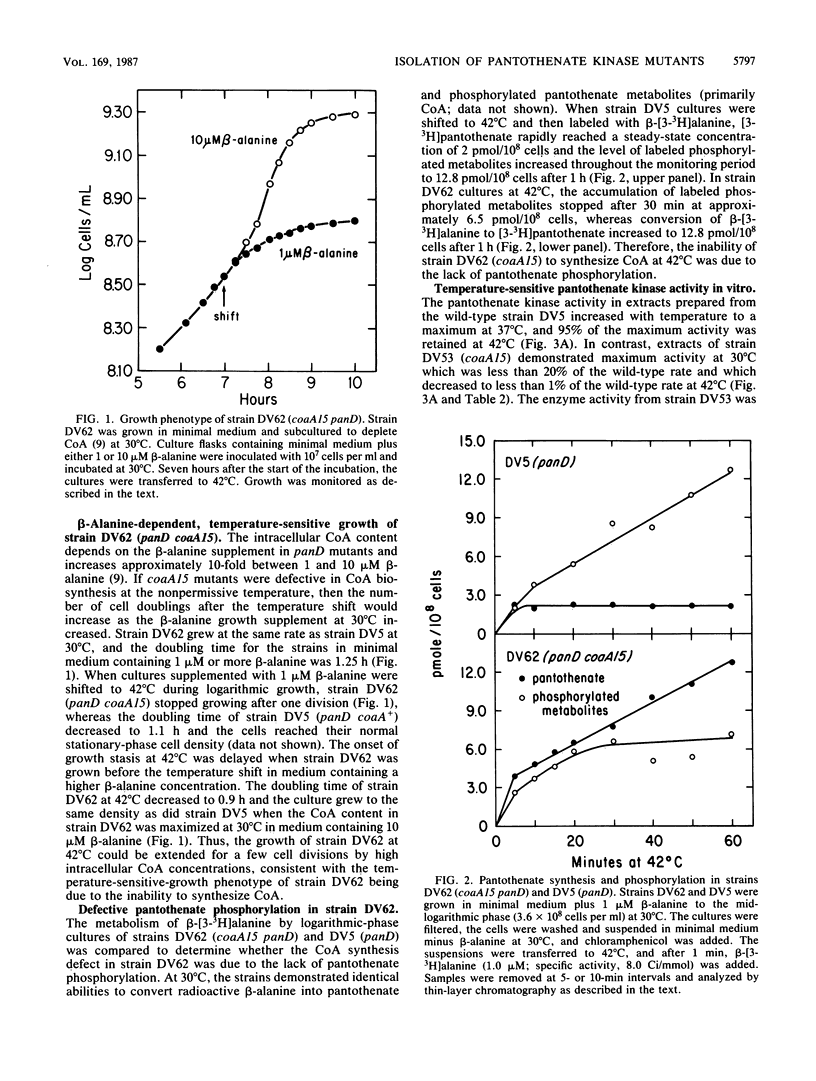
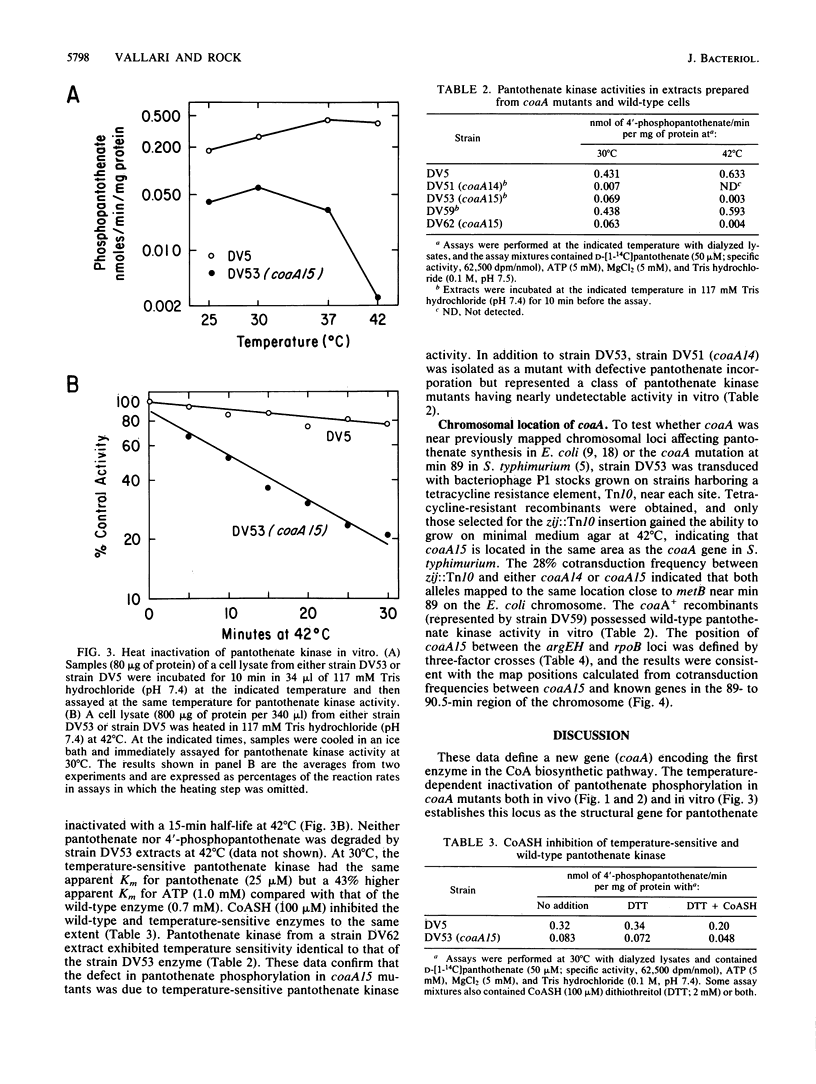
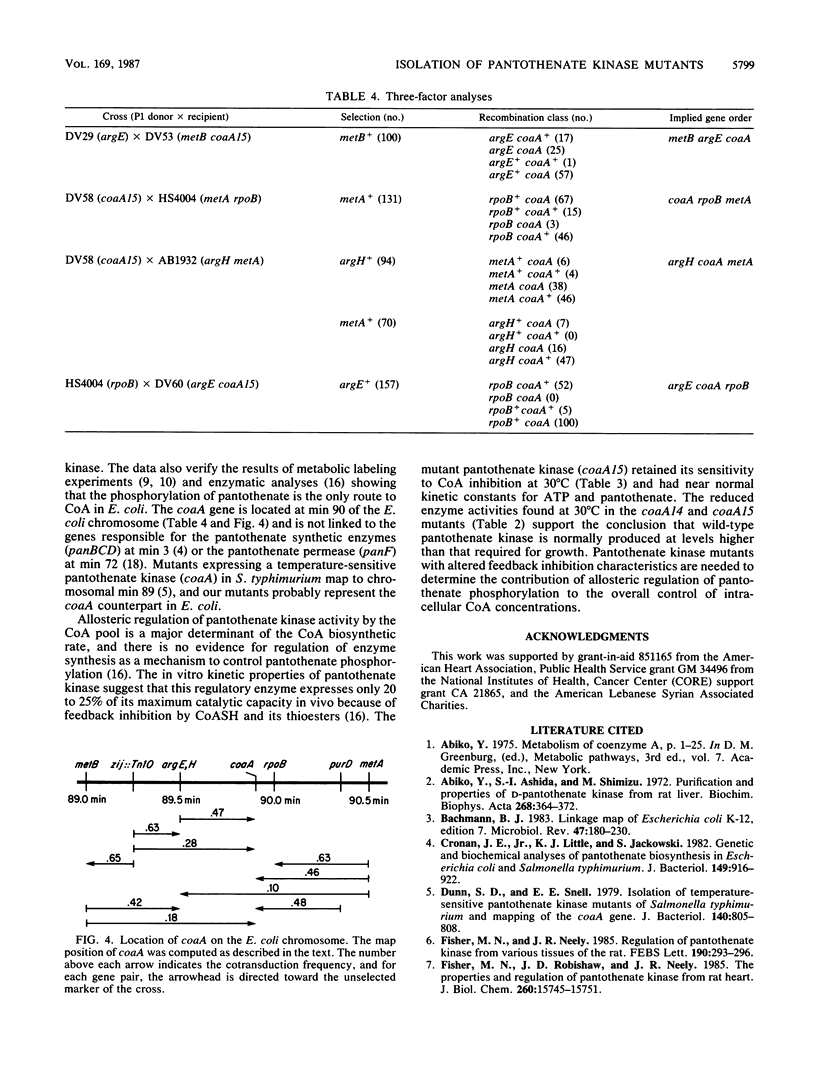
Escherichia coli mutants conditionally defective in the conversion of pantothenate to coenzyme A were isolated and characterized. The gene was designated coaA and localized between argEH and rpoB near min 90 of the chromosome. The coaA15(Ts) mutation caused a temperature-sensitive growth phenotype and temperature-dependent inactivation of pantothenate kinase activity assayed both in vivo and in vitro. At 30 degrees C, coaA15(Ts) extracts contained less than 20% of the wild-type pantothenate kinase activity; the kinase had near normal kinetic constants for the substrates ATP and pantothenate and was inhibited by coenzyme A to the same degree as the wild-type enzyme. These data define the coaA gene as the structural gene for pantothenate kinase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abiko Y., Ashida S. I., Shimizu M. Purification and properties of D-pantothenate kinase from rat liver. Biochim Biophys Acta. 1972 May 12;268(2):364–372. doi: 10.1016/0005-2744(72)90331-2. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Littel K. J., Jackowski S. Genetic and biochemical analyses of pantothenate biosynthesis in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1982 Mar;149(3):916–922. doi: 10.1128/jb.149.3.916-922.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S. D., Snell E. E. Isolation of temperature-sensitive pantothenate kinase mutants of Salmonella typhimurium and mapping of the coaA gene. J Bacteriol. 1979 Dec;140(3):805–808. doi: 10.1128/jb.140.3.805-808.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. N., Neely J. R. Regulation of pantothenate kinase from various tissues of the rat. FEBS Lett. 1985 Oct 14;190(2):293–296. doi: 10.1016/0014-5793(85)81303-x. [DOI] [PubMed] [Google Scholar]
- Fisher M. N., Robishaw J. D., Neely J. R. The properties and regulation of pantothenate kinase from rat heart. J Biol Chem. 1985 Dec 15;260(29):15745–15751. [PubMed] [Google Scholar]
- Halvorsen O., Skrede S. Regulation of the biosynthesis of CoA at the level of pantothenate kinase. Eur J Biochem. 1982 May;124(1):211–215. doi: 10.1111/j.1432-1033.1982.tb05927.x. [DOI] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Metabolism of 4'-phosphopantetheine in Escherichia coli. J Bacteriol. 1984 Apr;158(1):115–120. doi: 10.1128/jb.158.1.115-120.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Regulation of coenzyme A biosynthesis. J Bacteriol. 1981 Dec;148(3):926–932. doi: 10.1128/jb.148.3.926-932.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa T., Yoshida K., Furukawa K., Hosoki K. Feedback inhibition of pantothenate kinase by coenzyme A and possible role of the enzyme for the regulation of cellular coenzyme A level. J Biochem. 1972 Jun;71(6):1065–1067. doi: 10.1093/oxfordjournals.jbchem.a129854. [DOI] [PubMed] [Google Scholar]
- MUNKRES K. D., RICHARDS F. M. THE PURIFICATION AND PROPERTIES OF NEUROSPORA MALATE DEHYDROGENASE. Arch Biochem Biophys. 1965 Mar;109:466–479. doi: 10.1016/0003-9861(65)90391-7. [DOI] [PubMed] [Google Scholar]
- Robishaw J. D., Berkich D., Neely J. R. Rate-limiting step and control of coenzyme A synthesis in cardiac muscle. J Biol Chem. 1982 Sep 25;257(18):10967–10972. [PubMed] [Google Scholar]
- Smith C. M., Savage C. R., Jr Regulation of coenzyme A biosynthesis by glucagon and glucocorticoid in adult rat liver parenchymal cells. Biochem J. 1980 Apr 15;188(1):175–184. doi: 10.1042/bj1880175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vallari D. S., Jackowski S., Rock C. O. Regulation of pantothenate kinase by coenzyme A and its thioesters. J Biol Chem. 1987 Feb 25;262(6):2468–2471. [PubMed] [Google Scholar]
- Vallari D. S., Rock C. O. Isolation and characterization of Escherichia coli pantothenate permease (panF) mutants. J Bacteriol. 1985 Oct;164(1):136–142. doi: 10.1128/jb.164.1.136-142.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallari D. S., Rock C. O. Pantothenate transport in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1156–1161. doi: 10.1128/jb.162.3.1156-1161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallari D. S., Rock C. O. Preparation of high-specific-activity D-[3-3H]pantothenic acid. Anal Biochem. 1986 May 1;154(2):671–675. doi: 10.1016/0003-2697(86)90045-x. [DOI] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]


