Abstract
Thrombospondin-1 (TSP-1) is a naturally occurring inhibitor of angiogenesis that is able to make normal endothelial cells unresponsive to a wide variety of inducers. Here we use both native TSP-1 and small antiangiogenic peptides derived from it to show that this inhibition is mediated by CD36, a transmembrane glycoprotein found on microvascular endothelial cells. Both IgG antibodies against CD36 and glutathione-S-transferase–CD36 fusion proteins that contain the TSP-1 binding site blocked the ability of intact TSP-1 and its active peptides to inhibit the migration of cultured microvascular endothelial cells. In addition, antiangiogenic TSP-1 peptides inhibited the binding of native TSP-1 to solid phase CD36 and its fusion proteins, as well as to CD36-expressing cells. Additional molecules known to bind CD36, including the IgM anti-CD36 antibody SM∅, oxidized (but not unoxidized) low density lipoprotein, and human collagen 1, mimicked TSP-1 by inhibiting the migration of human microvascular endothelial cells. Transfection of CD36-deficient human umbilical vein endothelial cells with a CD36 expression plasmid caused them to become sensitive to TSP-1 inhibition of their migration and tube formation. This work demonstrates that endothelial CD36, previously thought to be involved only in adhesion and scavenging activities, may be essential for the inhibition of angiogenesis by thrombospondin-1.
Thrombospondin-1 (TSP-1)1 is one of a small number of molecules found naturally in vertebrates that can inhibit angiogenesis, the sprouting of new blood vessels from preexisting ones (Bouck et al., 1996). It is secreted at high levels by a variety of normal cells (Frazier, 1991; Lahav, 1993; Bornstein, 1995) and can decrease the density of the vasculature that forms during embryonic development (Stellmach et al., 1997) and wound healing in granulation tissue (Polverini, P.J., L.A. DiPietro, V.M. Dixit, R.O. Hynes, and J. Lawler. 1995. FASEB [Fed. Am. Soc. Exp. Biol.] J. 9:272a). In addition, TSP-1 can be an antiangiogenic barrier to the development of malignant tumors (Dameron et al., 1994; Weinstat-Saslow et al., 1994; Hsu et al., 1996; Volpert et al., 1997), which must induce a vigorous angiogenic response to grow and progress (Folkman, 1995b ).
TSP-1 is an extremely potent inhibitor of angiogenesis. It is effective at subnanomolar concentrations in vitro and able to prevent neovascularization in vivo in the rodent cornea and polyvinyl sponge assays (Good et al., 1990; Tolsma et al., 1993). The 50-kD central stalk region of the 180-kD subunit of homotrimeric TSP-1 is sufficient for its full antiangiogenic activity. Molecules that retain either the procollagen homology region or the properdin type 1 repeat region from this stalk also retain antiangiogenic activity (Tolsma et al., 1993; Volpert et al., 1995), and small peptides derived from each of these domains are independently able to block endothelial cell migration in vitro and neovascularization in vivo (Tolsma et al., 1993).
Soluble TSP-1 inhibits angiogenesis by interacting directly with endothelial cells. At concentrations below 20 nM, which is about forty times that seen in normal plasma (Saglio and Slayter, 1982; Dawes et al., 1983; Harker et al., 1983; Browne et al., 1996), it inhibits endothelial cells in vitro, blocking their proliferation (Bagavandoss and Wilkes, 1990; Good et al., 1990; Taraboletti et al., 1992; Vogel et al., 1993; Raychaudhury et al., 1994) and migration (Rastinejad et al., 1989; Good et al., 1990; Taraboletti et al., 1990, 1992; Tolsma et al., 1993, 1997; Volpert et al., 1995) and their ability to form lumens (Tolsma et al., 1997) and tubes (Iruela-Arispe et al., 1991; DiPietro et al., 1994; Canfield and Schor, 1995), although at higher concentrations it can be stimulatory for endothelial cell migration (Taraboletti et al., 1990; Tolsma et al., 1993; Gao et al., 1996).
TSP-1 can render endothelial cells refractory to a wide variety of inducers from prostaglandins to VEGF (Volpert et al., 1995), all of which stimulate cells through different receptors. To address the mechanism by which TSP-1 induces this unresponsiveness in the endothelial cell, we have sought a receptor through which it might act. The intact TSP-1 molecule binds to at least 12 different receptors (Bornstein, 1995), many of which are present on the surface of the endothelial cell. Some, including integrin αvβ3 (Lawler, 1993), integrin-associated protein (also referred to as CD47 [Gao et al., 1996]), and possibly low-density lipoprotein related receptor protein (Godyna et al., 1995; Mikhailenko et al., 1995), interact with TSP-1 motifs that lie outside the 50-kD stalk and thus seem unlikely to be involved in mediating the antiangiogenic activity of TSP-1. Although there are uncharacterized receptors present on endothelial cells that can bind to TSP-1 (Chen et al., 1996) and TSP-1–interacting molecules identified on other cell types that could also be present on endothelial cells (Yabkowitz and Dixit, 1991; Tuzinsky et al., 1993), CD36 currently appears to be the most likely candidate to mediate the angioinhibitory activity of TSP-1.
CD36, also called glycoprotein IV, is an 88-kD transmembrane glycoprotein with a large extracellular domain (Greenwalt et al., 1992). Originally found on platelets and monocytes, it is also expressed on the microvascular cells that give rise to neovascularization in vivo (Swerlick et al., 1992; Peltzbauer et al., 1993). CD36 is well known as an adhesion receptor for TSP-1 (Asch et al., 1987, 1993; Li et al., 1993; Pearce et al., 1995) and Plasmodium falciparum parasitized erythrocytes (Oquendo et al., 1989; Baruch et al., 1996), facilitates the binding of platelets to collagen, monocytes, and the subendothelium, and contributes to the activation of monocytes and platelets (Greenwalt et al., 1992). CD36 also serves as a scavenger receptor. It mediates the uptake of anionic phospholipids (Rigotti et al., 1995) and oxidized low-density lipoprotein (OxLDL) (Endemann et al., 1993; Nicholson et al., 1995; Nozaki et al., 1995), an activity that may contribute to the formation of foam cells involved in the genesis of artherosclerotic plaques, and plays a key role in the phagocytosis of apoptotic cells (Savill et al., 1992; Ren et al., 1995; Stern et al., 1996) and rod outer segments in the retina (Ryeom et al., 1996a ,b). Although not usually considered to be a signaling receptor, it coprecipitates from platelets and endothelial cells with several src-related kinases (Huang et al., 1991; Bull et al., 1994). Here we demonstrate that CD36 is an essential mediator of the antiangiogenic action of TSP-1 on endothelial cells in vitro. This is the first indication that engagement of CD36 can produce a biological response in endothelial cells and the first identification of a receptor able to mediate the effects of a broad spectrum inhibitor of angiogenesis like thrombospondin-1.
Materials and Methods
Reagents
FA6-152 monoclonal antibody against CD36 was purchased from Immunotech (Westbrook, ME), and SM∅ monoclonal antibody against CD36 and IgG1 and IgM control monoclonal antibodies, MOPC-21 and MOPC-104E, were from Sigma Chemical Co. (St. Louis, MO). OKM-5 monoclonal antibody against CD36 was kindly provided by Dr. Mary Makowski (Ortho Diagnostic Systems, Raritan, NJ). Before use, all monoclonal antibodies were extensively dialyzed against DME using a 30-kD Centricon® concentrator (Amicon Corp., Beverly, MA).
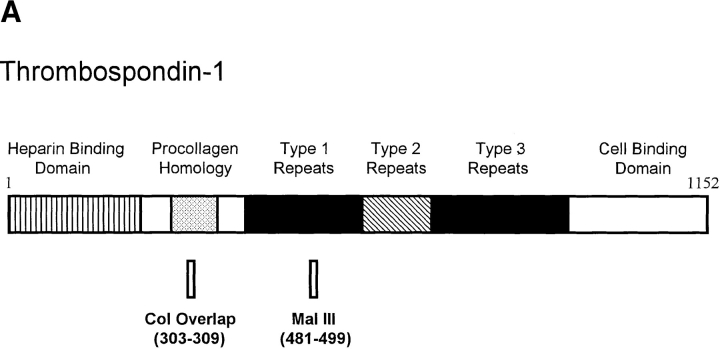
Purified human platelet TSP-1 was kindly provided by Jack Lawler (Harvard University, Cambridge, MA). TSP-1 peptides were synthesized, purified, and dialyzed as previously described (Tolsma et al., 1993) and included Col overlap, NGVQYRN representing TSP-1 amino acid residues 303–309; Mal III, SPWDIASVTAGGGVQKRSK representing TSP-1 amino acid residues 481–499 with the cysteines present in the native molecule replaced with alanines; and Mal III variant, SPWDIASTSAGGGVQRSK, containing Mal III residues with an altered VTCG sequence. The position of these peptides in the intact TSP-1 molecule is shown in Fig. 1. Angiostatin was kindly provided by Michael O'Reilly and Judah Folkman (Harvard University). Recombinant human basic fibroblast growth factor (bFGF) was purchased from R & D Systems Inc. (Minneapolis, MN), human type I collagen was from Collaborative Biomedical Products (Bedford, MA), and human LDL was from Sigma Chemical Co. LDL was oxidized by dialyzing 200 μg/ml LDL against 5 μM CuSO4 in PBS for 24 h at 37°C (Nicholson et al., 1995).
Figure 1.
The relationship of small TSP-1 peptides to the 180-kD monomer of TSP-1 and of GST–CD36 fusion proteins to the whole CD36 receptor protein. Numbers indicate amino acid residues present in the peptide or fusion protein. Shading on the whole CD36 molecule defines the minimal region of CD36 required for binding to TSP-1 (Pearce et al., 1995). Actual peptide sequences and detailed description of the fusion proteins are included in Materials and Methods.
Preparation of Glutathione-S-transferase–CD36 Fusion Proteins
CD36–glutathione-S-transferase (GST) recombinant fusion proteins spanning ∼98% of the human CD36 sequence were previously generated in a bacterial expression system (Pearce et al., 1995). In this study, we used fusion proteins containing the TSP-1 binding region and spanning amino acids 67–157, 93–120, and 93–298. As a control, we used a fusion protein unable to bind TSP-1 spanning CD36 amino acids 298–439. For the relative positions of these peptides on CD36, see Fig. 1. As described previously (Pearce et al., 1995), all plasmid constructs were mapped and insertion sites sequenced to confirm that the fusion protein sequences were correct and in frame. Generated fusion proteins were examined by SDS-PAGE, Western blot, gel filtration chromatography, and ELISA to confirm size and document CD36 immunoreactivity. All molecular weights as determined by gel filtration chromatography under nondenaturing conditions varied no more than 5% from calculated values (Pearce et al., 1995). These assays also showed that the fusion proteins did not form dimers or large multimers.
Binding Studies
Cell binding assays were performed with Bowes melanoma cells stably transfected with human CD36 cDNA, or with a control plasmid, prepared and maintained as previously described (Silverstein et al., 1992). Cells were allowed to attach to 12-well plates. Radiolabeled TSP-1 was prepared as previously described (Pearce et al., 1995) with specific activity ranging from 0.5–1 mCi/mg. Inhibition studies were carried out by combining 125I–TSP-1 (20 μg/ml) with increasing concentrations of peptide (0.1–1000 μM). After incubation for 2 h at 37°C, the cells were washed five times with cold PBS and lysed with 0.1 N NaOH. The amount of bound radioactivity was determined by gamma counting. The peptide LYPQHKT, obtained from a domain of CD36 that does not bind TSP, was used as a control. IC50's were calculated using the curve fitting software program Enzfitter (Elsevier Biosoft, Cambridge, UK).
Solid phase binding assays were used to measure TSP-1 interactions with CD36 or CD36–GST fusion proteins in the presence of TSP-1 peptides. CD36 or CD36–GST fusion protein was immobilized on wells in a detachable 96-microwell plate by overnight incubation at 4°C. CD36 was adsorbed at 4 μg/ml in PBS, while fusion proteins were adsorbed at 10 μg/ ml in carbonate buffer (100 mM NaHCO3, 1 mM Mg Cl2, 0.02% NaN3, pH 9.8). Total protein coating the wells ranged from 200–280 ng. Wells were washed three times with 20 mM Tris, 150 mM NaCl, pH 7.4, containing 0.05% Tween-20 (TBS-T) and then blocked with TBS-T containing 0.5% BSA. Radiolabeled TSP-1 (20 μg/ml) along with two concentrations of peptide (at the K d and five times the K d as predicted by the cell binding studies) were added in TBS-T, and the mixture was incubated for 2 h at 22°C. Wells were then washed thoroughly three to four times with TBS-T and dried, and bound radioactivity was measured by gamma counting. The addition of 5 mM EDTA was used to determine nonspecific binding.
Migration Assays
Bovine adrenal capillary endothelial cells (BCECs), kindly provided by Judah Folkman, were grown in DME with 10% donor calf serum (Flow Laboratories, McLean, VA) and 1% endothelial cell mitogen (Biomedical Technologies, Inc., Stoughton, MA) and used at passage 15. Human dermal microvascular endothelial cells (HMVECs) were purchased from Clonetics (San Diego, CA), grown in Clonetics' endothelial cell growth media, and used between passages 6 and 9. Human umbilical vein endothelial cells (HUVECs), generously provided by Dr. L. Cornelius (Washington University, St. Louis, MO), were grown in 199 Earles medium with 20% FCS and 10 μg/ml endothelial cell mitogen and used between passages 6–11.
Migrations were performed as previously described (Polverini et al., 1991). Briefly, confluent flasks of endothelial cells were starved overnight in control medium (DME containing 0.1% BSA). Cells were harvested, resuspended in control medium, and plated at 3 × 104 cells/well on the bottom side of a gelatinized 5 μM (for BCECs) or 8 μM (for HMVECs and HUVECs) porous membrane (Nucleopore Corp., Pleasanton, CA) in an inverted modified Boyden chamber. Cells were allowed to attach to the membrane for 2 h at 37°C, after which the chamber was reinverted, test samples were added to the top wells, and cells were allowed to migrate towards the top well for 4 h at 37°C. Membranes were removed, fixed, and stained, and the number of cells migrating to the top side of the membrane per 10 high-power fields was counted. Test samples containing GST fusion proteins were first preincubated for 2 h at 4°C and then warmed to room temperature before addition to the chamber. With the exception of SM∅, monoclonal antibodies, when used, were added to both the upper and lower wells. Because OKM-5 is unable to recognize bovine CD36 (Ockenhouse et al., 1989), human HMVECs were used instead of bovine BCECs for migrations with anti-CD36 monoclonal antibodies. Control medium alone was used as a negative control to measure background resulting from random cell movement. Samples in each experiment were tested in quadruplicate, and experiments were repeated at least twice. Data presented in figures have been normalized to maximum migration, where 100% was calculated as the migration towards bFGF minus the background migration towards control medium alone. Background was consistently less than 60% of migration towards bFGF. Negative percentages appear when test samples suppressed random cell movement. Where indicated, statistical significance between sample means was determined using a two-tailed t test on initial raw data within a single experiment, before background subtraction and normalization.
Transfections and Flow Cytometry
The plasmid pCDM8-CD36 was a kind gift of Dr. B. Seed (Massachusetts General Hospital, Boston, MA). The HindIII-NotI fragment of CD36 was ligated to the vector pcDNAneo (Invitrogen, San Diego, CA) predigested with HindIII and NotI to produce a CD36 mammalian expression vector containing an enhancer/promoter sequence of the immediate early gene of the human cytomegalovirus and a SV-40 polyadenylation signal. HUVECs at passage 2 were transfected with this pcDNAneo-CD36 using lipofectin reagent (GIBCO BRL, Gaithersburg, MD). 30 μg of plasmid DNA was preincubated in 100 μl lipofectin for 15 min at room temperature. Cells grown to 30–50% confluency were washed with serum-free media and refed with 4 ml of Opti-MEM medium (GIBCO BRL) plus the plasmid/lipofectin mix. The cells were then incubated for 5 h in 5% CO2 at 37°C, subsequently rinsed twice with growth medium, allowed to recover in normal growth medium for 2 d, and finally split into selection medium with 100 μg/ml G418. Single colonies were picked with cloning rings (Bellco Glass, Inc., Vineland, NJ), expanded, and screened.
CD36-positive clones were characterized and analyzed by flow cytometric analysis. Cells were washed twice with cold PBS, incubated with anti-CD36 monoclonal antibody SM∅ or with an IgM control at 5 μg/ml for 45 min on ice, washed, incubated with FITC-conjugated goat anti–mouse IgM (5 μg/ml) for an additional 45 min on ice, washed again, and finally analyzed on a Becton-Dickinson FACScan® flow cytometer (Mountain View, CA).
Tube Formation Assay
Matrigel was purchased from Biomedical Technologies, Inc. 150 μl of Matrigel matrix was added to each well of a 48-well plate and incubated at 37°C for 30 min to allow for gel formation. HUVECs at 80–90% confluency were trypsinized, washed twice with PBS, plated on the Matrigel at 3.5 × 104 cells/well, incubated in 5% CO2 at 37°C for 24 h, and then photographed.
Results
CD36 Fusion Proteins Blocked the Inhibitory Activity of TSP-1 and Its Antiangiogenic Peptides
To determine if an interaction between CD36 and TSP-1 could block the antiangiogenic activity of TSP-1, soluble GST–CD36 fusion proteins were tested for their ability to block the inhibition of bovine capillary endothelial cell migration by TSP-1. This assay measures the migration of cultured endothelial cells toward a known angiogenic factor (bFGF) and consistently parallels angiogenic activity seen in vivo (Folkman and Klagsbrun, 1987; Klagsbrun and D'Amore, 1991; Bouck et al., 1996). TSP-1 was previously shown to interact in a specific, saturable, and reversible manner with a minimal region of CD36 encompassing amino acids 93–120 (Pearce et al., 1995). As shown in Fig. 2 A, GST–CD36 fusion proteins FP93-120 and FP93-298, both of which contained the TSP-1 binding domain, were able to block TSP-1 inhibition of bFGF-induced migration in a dose-dependent fashion, whereas GST alone (data not shown) and fusion protein FP298-439, which lacked the TSP-1 binding domain (Fig. 2 A), were ineffective.
Figure 2.
Interference with the activity of TSP-1 and its antiangiogenic peptides by soluble GST–CD36 fusion proteins. Increasing concentrations of CD36 fusion proteins that contain a TSP-1 binding site, FP93-120 (circles) or FP93-298 (triangles), or a CD36 fusion protein that lacks a TSP-1 binding site, FP298-439 (squares), were preincubated for 2 h at 4°C with (A) 2 nM TSP-1, (B) 10 μM Mal III peptide, (C) 30 μM Col overlap peptide, or (D) control media. Each mixture was then tested for the ability to block bovine capillary endothelial cell migration towards bFGF (solid symbols) or influence background migration in the absence of bFGF (D, open symbols). Data, accumulated from nine experiments, are reported as a percentage of maximum migration, where 100% represents the number of cells migrating towards the inducer bFGF alone, and 0% corresponds to the number of cells migrating randomly in the absence of inducer (Bkgd). 100% varied between experiments from 32 to 81 cells migrated/10 high-power fields. Bars indicate standard errors.
To more stringently link the TSP-1–CD36 interaction with inhibition of angiogenesis, blocking studies with the GST–CD36 fusion proteins were repeated with small TSP-1 peptides known to inhibit endothelial cell migration and neovascularization in vivo (Tolsma et al., 1993). Concentrations of inhibiting TSP-1 peptides were deliberately chosen to be less than 100% effective so that both positive and negative effects on migration could be observed. CD36 fusion proteins containing TSP-1 binding sites interfered with the antiangiogenic activity of Mal III, a 19-mer containing a homologue of the CSVTCG sequence previously reported to interact with CD36 (Asch et al., 1992; Leung et al., 1992; Li et al., 1993), and of Col overlap, a 7-mer lacking the CSVTCG sequence (Figs. 2, B and C). Loss of inhibition could not be attributed to induction of migration by the fusion proteins themselves as each was neutral when tested alone in this assay (Fig. 2 D). The inhibitory activities of the peptides were quenched only by CD36 fusion proteins that also bound TSP-1 (Figs. 2, B and C).
Antiangiogenic TSP-1 Peptides Physically Associated with CD36
The Mal III and Col overlap peptides physically interacted with CD36 because both were able to competitively displace 125I –TSP-1 from CD36 expressed in its natural context on the surface of CD36 cDNA-transfected Bowes melanoma cells. TSP-1 peptides Mal III and Col overlap, as well as the Mal III variant that lacks the VTCG motif but still inhibits endothelial cell migration (Tolsma, S., and N. Bouck, personal communication), were all able to inhibit binding of TSP-1 to CD36 (Fig. 3 A). Inhibition constants (IC50's) were 8.60 ± 1.12 μM for Mal III, 30.08 ± 8.88 μM for Col overlap, and 15.78 ± 2.08 μM for Mal III variant. A control peptide did not inhibit binding demonstrating specificity. Melanoma cells not transfected with CD36 do not bind TSP-1 (Silverstein et al., 1992). The calculated IC50 of each peptide closely approximated its previously reported ED50 for inhibition of endothelial cell migration (Tolsma et al., 1993).
Figure 3.
Inhibition of TSP-1 binding to CD36 and its fusion proteins by antiangiogenic TSP-1 peptides. (A) Binding of 125I–TSP-1 (20 μg/ ml) to a confluent monolayer of Bowes melanoma cells expressing CD36 was determined in the presence of increasing concentrations of antiangiogenic TSP-1 peptides Mal III (circles, lowest curve), Mal III variant (triangles, second lowest curve), and Col overlap (squares, third lowest curve) or of control peptide LYPQHKT (diamonds, top curve). Data were normalized as a percentage of TSP-1 bound under control conditions. (B) The effects of TSP-1 peptides Mal III and Col overlap on the binding of 125I–TSP-1 (20 μg/ml) to solid phase CD36 and CD36 fusion proteins FP67-157 and FP93-120, which contain a TSP-1 binding site, and to FP298-439, which does not, are shown. Nonspecific binding was determined in the presence of 5 mM EDTA. Bars indicate standard error (n = 3 for A and n = 5 for B).
To demonstrate that TSP-1 peptides interacted only with CD36 fusion proteins containing the TSP-1 binding domain (Pearce et al., 1995), their ability to block 125I–TSP-1 binding to immobilized CD36 and CD36–GST fusion proteins was tested (Fig. 3 B). Mal III at 10 μM reduced the specific binding of TSP-1 to CD36 and to two CD36–GST fusion proteins by 53–65%. Col overlap was similarly effective at inhibiting the binding of TSP-1 to intact CD36 and to the longer fusion protein FP67-157. The CD36 fusion protein FP298-439, which lacks the TSP binding site, showed only background binding equal to that seen in the presence of 5 mM EDTA. The clear dose response relationship seen for peptide binding to CD36 expressed on cells in Fig. 3 A was much less evident in the cell-free binding experiments (Fig. 3 B), likely caused in part by immobilization of CD36 and its fusion proteins on plastic and in part by the use of high peptide concentrations where a significant dose response effect might not be expected. There is an interesting discrepancy in that the Col overlap peptide failed to block TSP-1 binding to the CD36–GST fusion protein containing the minimal TSP-1 binding domain (FP93-120; Fig. 3 B), although this same fusion protein blocked the inhibitory effects of Col overlap on endothelial cell migration (Fig. 2 C). This discrepancy suggests that Col overlap has a much lower affinity for solid phase FP93-120 than soluble FP93-120, perhaps because of constraints imposed on this short fusion protein when bound to plastic.
CD36 Antibodies Specifically Blocked the Inhibitory Activity of TSP-1 and Its Antiangiogenic Peptides
To demonstrate more directly that TSP-1 inhibits angiogenesis via an interaction with CD36, two anti-CD36 monoclonal antibodies, OKM-5 and FA6-152, known to map to an immunodominant epitope on the extracellular region of CD36 (Daviet et al., 1995) as well as to physically displace TSP-1 from CD36 (Asch et al., 1987; Kieffer et al., 1989), were tested. Both antibodies blocked the inhibition of bFGF-induced migration of HMVECs by TSP-1 (Fig. 4 A) and by TSP-1 peptides (Fig. 4 B). This block was specific to TSP-1 as neither antibody affected the inhibition of endothelial cell migration by angiostatin (Fig. 4 A), another well-established inhibitor of endothelial cell migration in vitro (Gately et al., 1996) and of angiogenesis in vivo (O'Reilly et al., 1994). An isotype-matched control monoclonal antibody was without effect in the assay (Fig. 4 A). The two anti-CD36 antibodies were also able to block TSP-1 inhibition of migration induced by angiogenic molecules other than bFGF. For example, antibody FA6-152 blocked TSP-1 inhibition of endothelial cell migration induced by either scatter factor (Lamszus et al., 1996) or vascular endothelial growth factor (Koch et al., 1994) (data not shown).
Figure 4.
Interference with the activity of TSP-1 and its antiangiogenic peptides by monoclonal IgG antibodies against CD36. Monoclonal IgG antibodies against CD36, FA6-152, and OKM-5 or an isotype-matched control were tested at 10 μg/ml for ability to block the inhibition of human microvascular endothelial cell migration towards bFGF by (A) TSP-1 (2 nM) or (B) TSP-1 peptides Mal III (30 μM) or Col overlap (50 μM). Angiostatin (2 μg/ml) served as a control inhibitor. Data from three separate experiments were normalized and reported as in Fig. 1. 100% varied between experiments from 32 to 53 cells migrated/10 high-power fields. *Samples significantly different from parallel condition using control media, P < 0.02.
Engagement of CD36 by Other Ligands Also Inhibited Endothelial Cell Migration
SM∅, an anti-CD36 monoclonal antibody of the IgM class, mimicked the activity of TSP-1 and inhibited bFGF-induced HMVEC migration, while an isotype-control monoclonal antibody had no significant effect (Fig. 5 A). The high valency of this pentameric IgM presumably allowed for oligomerization of receptors that did not occur when divalent anti-CD36 antibodies of the IgG class were used (Fig. 4). Similar differential antibody effects of IgGs and IgMs are also seen with FAS and TNF receptors (Nagata, 1997). Two other known ligands of CD36, human collagen I and OxLDL, also inhibited bFGF-induced HMVEC migration (Fig. 5 B) and showed maximal inhibitory activity at concentrations similar to those at which they saturably bind CD36 (Tandon et al., 1989; Endemann et al., 1993). These effects were mediated via CD36, for they were blocked by anti-CD36 monoclonal antibodies OKM-5 or FA6-152 (Fig. 5 B). Inhibition was concentration dependent, as both collagen I and OxLDL were ineffective at inhibiting migration at concentrations below 0.1 nM (data not shown). Unoxidized LDL, which is unable to bind CD36 (Endemann et al., 1993), failed to influence HMVEC migration (Fig. 5 B).
Figure 5.
Inhibition of endothelial cell migration by additional CD36 ligands. (A) A murine anti-CD36 IgM monoclonal antibody SM∅ and an isotype-matched control monoclonal antibody were tested at 1 μg/ml for ability to inhibit human microvascular endothelial cell migration towards bFGF. Data were normalized and reported as in Fig. 1. *Significant inhibition compared with bFGF tested alone, P < 0.001. (B) Human collagen I, LDL, and OxLDL were tested at 2 μg/ml in the presence or absence of 10 μg/ml of monoclonal antibodies against CD36 for ability to inhibit migration of human microvascular cells. Data from two experiments were normalized and reported as in Fig 1. When tested alone, collagen I, OxLDL, and LDL had no significant effect on migration. 100% varied between experiments from 29 to 42 cells migrated/10 high-power fields. *Samples significantly different from bFGF tested with control media, P < 0.001.
CD36-transfected HUVECs Became Responsive to TSP-1 Inhibition
CD36 is typically expressed on human microvascular endothelial cells such as HMVECs, but it is absent from large vessel endothelial cells such as HUVECs (Swerlick et al., 1992; Peltzbauer et al., 1993), thus providing a useful way to assess CD36 function. HUVECs negative for CD36 as determined by FACS® (Fig. 6), by immunoprecipitation followed by Western blot, and by Northern blot (data not shown) were transfected with a CD36 expression vector, and a series of clones expressing increasing levels of CD36 as judged by FACS® and by Western blots were selected (Fig. 6). It was noted that clones expressing any appreciable level of CD36 had slower growth rates than parental HUVECs. For example, the relative increases in cell number after 8 d of culture for each clone were HUVEC, 6.2; clone 35, 5.5; clone 31, 2.1; and clone 36, 1.4. The transfectants also exhibited a progressively aberrant, very highly spread morphology as seen in the middle panels of Fig. 6 (photographed at confluency). When plated in Matrigel, the degree of inhibition of tube formation was correlated with level of CD36 expression (Fig. 6). In total, 30 clones expressing various levels of CD36 were characterized and found to have growth impairment and morphological changes proportional to their CD36 levels. An additional five clones were analyzed for tube formation, and they fit the pattern seen in Fig. 6. All of the CD36-positive clones maintained high levels of TSP-1 expression as determined by Western blotting of serum-free culture media collected over 24 h. Relative amounts of TSP-1 as determined by densitometry were HUVEC, 6.8; clone 35, 8.1; clone 31, 6.3; and clone 36, 6.0.
Figure 6.

Sensitivity to TSP-1 inhibition of tube formation induced by CD36 expression in CD36-deficient HUVECs. The first column shows FACS® analysis for CD36 expression performed on untransfected HUVECs, a transfectant expressing very low levels of CD36 (clone 35), and two transfectants expressing higher levels of CD36 (clones 31 and 36). Fluorescence is plotted vs. cell number. The dotted line in the clones reproduces the HUVEC CD36 null pattern. The morphology of each of the transfectants grown in two-dimensional cell culture is shown in the second column. In the third column, tube formation in a three-dimensional culture of Matrigel is pictured for each line.
When tested in a migration assay, clones 31 and 36 were sensitive to TSP-1 inhibition, and this inhibition was abrogated by a CD36-blocking antibody (Fig. 7). Clone 35, which expressed only very low levels of CD36 (FACS® analysis in Fig. 6 and Western blotting with anti-CD36 mAb Mo-91, not shown), was refractory to TSP-1 inhibition (Figs. 6 and 7). Thus, introduction of CD36 into HUVECs rendered them susceptible to inhibition of chemotaxis by TSP-1 and induced, either directly or indirectly, changes in growth rate, morphology, and ability to organize into tubular structures in Matrigel.
Figure 7.
Sensitivity to TSP-1 inhibition of migration after transfection of HUVECs with CD36. Clones described in Fig. 5 were tested for sensitivity to inhibition of migration by 2 nM TSP-1 in the presence and absence of 10 μg/ml anti-CD36 monoclonal antibody OKM-5. Data from four separate experiments were normalized and reported as in Fig 1. 100% varied between experiments from 37 to 82 cells migrated/10 high-power fields. *Samples that differ significantly from bFGF tested alone, P < 0.02.
Discussion
CD36 is necessary for the inhibition of endothelial cell migration and tube formation by TSP-1. Soluble GST–CD36 proteins blocked the inhibition of migration by either intact TSP-1 or small TSP-1 peptides only when they contained the TSP-1 binding site. Blocking antibodies against CD36 prevented TSP-1 from inhibiting migration, while an IgM monoclonal antibody against CD36 mimicked TSP-1 and directly inhibited migration. Large vessel endothelial cells that lacked CD36 became sensitive to TSP-1 inhibition of both migration and tube formation after CD36 transfection. The inhibition mediated by CD36 in vitro likely reflects the in vivo situation because inhibition of migration is a consistent predictor of inhibition of neovascularization in vivo (Folkman and Klagsbrun, 1987; Klagsbrun and D'Amore, 1991; Bouck et al., 1996). Furthermore, experiments to be reported elsewhere show that inhibition of corneal neovascularization by TSP-1 but not by angiostatin is defective in CD36 null animals (Febbraio, M., O.V. Volpert, N.P. Bouck, and R.L. Silverstein, unpublished data).
These results indicate that the antiangiogenic activity of TSP-1 is receptor mediated. They are not consistent with TSP-1 inhibiting angiogenesis by sequestering inducers, as has been suggested by the finding that TSP-1 can bind directly to inducers of angiogenesis, such as scatter factor (Lamszus et al., 1996). In fact, in this study TSP-1 inhibition of scatter factor–induced migration could be completely blocked by an antibody to CD36. Our results are also not consistent with models suggesting that TSP-1 inhibits bFGF-induced angiogenesis by competing with it for binding proteoglycans on endothelial cells, as suggested by experiments with heparin-binding TSP-1 peptides (Vogel et al., 1993), because migration induced by bFGF was effectively blocked in the absence of TSP-1 by agents that directly activated CD36, and inhibition by TSP-1 was antagonized by antibodies that blocked CD36 engagement.
While CD36 has often been implicated in adhesion and scavenging (Greenwalt et al., 1992), data presented here show that CD36 expressed on endothelial cells is also a signaling receptor able to trigger a biological response. The response in this case is one that generates an inhibitory signal that blocks a positive response to inducers of angiogenesis. It is reminiscent of receptor-mediated negative signals to which immune cells are particularly sensitive (Scharenberg and Kinet, 1996). Such signals often require a coreceptor and are mediated by src family kinases. It is not known if CD36 acts alone or in concert with an unidentified coreceptor or other surface molecule, but it does coprecipitate from endothelial cells in association with the src family kinase p59fyn, and possibly other src family kinases (Bull et al., 1994). It is also not yet clear how a signal emanating from CD36 might block the variety of stimulatory signaling cascades initiated by many different inducers of angiogenesis. However, the cytoplasmic domain of CD36 has been seen to associate with focal adhesion kinase (Sheibani, N., R. Zhong, and W.A. Frazier, manuscript in preparation), a kinase that can be essential for the stimulation of cell movement (Cary et al., 1996; Gilmore and Romer, 1996). If activation of CD36 were to disable focal adhesion kinase and thereby prevent the adhesion-associated tyrosine phosphorylation that may be essential for endothelial cell motility (Williams et al., 1996), the effectiveness of a wide variety of inducers of angiogenesis would be severely compromised.
Although the CSVTCG motif on TSP-1 has previously been thought to mediate its interactions with CD36 (Asch et al., 1992; Catimel et al., 1992; Li et al., 1993), data presented here show that an additional TSP-1 motif can interact with CD36. The Col overlap peptide lacking CSVTCG was antiangiogenic, was able to displace TSP-1 from CD36-transfected melanoma cells, and was rendered inactive by CD36 fusion proteins. A Mal III variant also lacking CSVTCG was antiangiogenic and able to displace TSP-1 from CD36-expressing cells. These peptides share a central GVQXR sequence that could represent a second CD36 binding motif. Two-step binding of TSP-1 to CD36 has been postulated by others (Leung et al., 1992). Perhaps CSVTCG facilitates an initial interaction between TSP-1 and CD36 and GVQXR mediates a signaling response. Such a role would fit well with our observation that the Mal III peptide, which contains both motifs, had a higher affinity for CD36 and is a more potent inhibitor of migration (Tolsma et al., 1993) than Col overlap, which lacks CSVTCG.
The identification of CD36 as a mediator of the inhibitory effects of TSP-1 helps to explain the unusual biphasic dose response curve generated when TSP-1 effects on endothelial cell migration are measured. TSP-1 inhibits endothelial cell migration at low concentrations less than 20 nM, yet stimulates migration at higher concentrations (Taraboletti et al., 1990; Tolsma et al., 1993; Gao et al., 1996), a fact that has led to much confusion about its ultimate activity. Recent work has shown that a blocking antibody against the integrin-associated protein (IAP) prevents higher concentrations of TSP-1 from inducing endothelial cell migration (Gao et al., 1996). Thus, the biphasic dose response curve for TSP-1 can be explained as resulting from the sum of activities of two distinct receptors, inhibitory CD36 and stimulatory IAP. CD36 mediates the inhibitory effects of TSP-1 at lower concentrations. The ability of CD36 to mediate phagocytosis (Ryeom et al., 1996a ,b) and the rapid degradation of lipids (Nozaki et al., 1995) suggest that it may be rapidly cleared from the cell surface upon engagement with TSP-1. Thus, at high concentrations of TSP-1, CD36 may be sufficiently depleted to allow IAP to act unopposed, resulting in the stimulation of endothelial cell migration.
The binding activity of CD36 expressed on platelets has been shown to be regulated by the extracellular phosphorylation state of its ectodomain, with increasing phosphorylation decreasing its binding to TSP-1 and increasing its affinity for collagen and vice versa (Asch et al., 1993). The in vitro sensitivity of CD36 to both ligands suggests that cultured endothelial cells have both phosphorylated and unphosphorylated CD36 molecules on their surface. In vivo it is possible that changes in CD36 phosphorylation by extracellular phosphatases and kinases could regulate the effect of both TSP-1 and other CD36 ligands on endothelial cells. This may be of particular importance at sites of platelet secretion and inflammation.
A variety of antiangiogenic compounds are now entering clinical trials as anticancer agents (Folkman, 1995a ; Gradishar, 1997). The identification of CD36 as an inhibitory signaling receptor for TSP-1 could be useful in the design and discovery of additional pharmacologic agents that can inhibit pathologic neovascularization.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health/National Cancer Institute (NIH/NCI) CA52750 and CA64239 (to N.P. Bouck) and CA65872 (to W.A. Frazier); NIH/NCI Institutional Training Grant 5T32CA09569 and Chicago Baseball Charities (to D.W. Dawson); NIH HL46403 and EY10967 and the Charles Fogarty Trust (to R.L. Silverstein); and a grant-in-aid from the American Heart Association, New York City Affiliate, and the Dorothy Rodbell Cohen Foundation (to S.F.A. Pearce).
Abbreviations used in this paper
- BCEC
bovine capillary endothelial cell
- bFGF
basic fibroblast growth factor
- FP
fusion protein
- GST
glutathione-S-transferase
- HMVEC
human microvascular endothelial cell
- HUVEC
human umbilical vein endothelial cell
- IAP
integrin-associated protein
- (Ox)LDL
(oxidized) low-density lipoprotein
- TSP-1
thrombospondin-1
Footnotes
Address all correspondence to Noël P. Bouck, Cancer Center, Northwestern University Medical School, 303 East Chicago Ave., Chicago, IL 60611. Tel.: (312) 503-5934. Fax: (312) 908-1372. E-mail: n-bouck@nwu.edu
References
- Asch AS, Barnwell J, Silverstein RL, Nachman RL. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987;79:1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch AS, Silbiger S, Heimer E, Nachman RL. Thrombospondin sequence motif (CSVTCG) is responsible for CD36 binding. Biochem Biophys Res Commun. 1992;182:1208–1217. doi: 10.1016/0006-291x(92)91860-s. [DOI] [PubMed] [Google Scholar]
- Asch AS, Liu I, Briccetti FM, Barnwell JW, Kwakye-Berko F, Dokun A, Goldberger J, Pernambuco M. Analysis of CD36 binding domains: ligand specificity controlled by dephosphorylation of an ectodomain. Science (Wash DC) 1993;262:1436–1440. doi: 10.1126/science.7504322. [DOI] [PubMed] [Google Scholar]
- Bagavandoss P, Wilkes JW. Specific inhibition of endothelial cell proliferation by thrombospondin. Biochem Biophys Res Commun. 1990;170:867–872. doi: 10.1016/0006-291x(90)92171-u. [DOI] [PubMed] [Google Scholar]
- Baruch DI, Gormely JA, Ma C, Howard RJ, Pasloske BL. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intracellular adhesion molecule 1. Proc Natl Acad Sci USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N, Stellmach V, Hsu S. How tumors become angiogenic. Adv Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- Browne PV, Mosher DF, Steinberg MH, Hebbel RP. Disturbance of plasma and platelet thrombospondin levels in sickle cell disease. Am J Hematol. 1996;51:296–301. doi: 10.1002/(SICI)1096-8652(199604)51:4<296::AID-AJH8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Bull HA, Brickell PM, Dowd PM. src-related protein tyrosine kinases are physically associated with the surface antigen CD36 in human dermal microvascular endothelial cells. FEBS Lett. 1994;351:41–44. doi: 10.1016/0014-5793(94)00814-0. [DOI] [PubMed] [Google Scholar]
- Canfield AE, Schor AM. Evidence that tenascin and thrombospondin-1 modulate sprouting of endothelial cells. J Cell Sci. 1995;108:797–809. doi: 10.1242/jcs.108.2.797. [DOI] [PubMed] [Google Scholar]
- Cary LA, Chang JF, Guan J-L. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- Catimel B, Leung L, El H, Ghissasi, Mercier N, McGregor J. Human platelet glycoprotein IIIb binds to thrombospondin fragments bearing the COOH-terminal region, and/or the type I repeats (CSVTCG motif), but not to the NH2-terminal heparin-binding region. Biochem J. 1992;284:231–236. doi: 10.1042/bj2840231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z-S, Pohl J, Lawley TJ, Swerlick RA. Human microvascular endothelial cells adhere to thrombospondin-1 via an RGD/CSVTCG domain independent mechanism. J Invest Dermatol. 1996;106:215–220. doi: 10.1111/1523-1747.ep12340475. [DOI] [PubMed] [Google Scholar]
- Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science (Wash DC) 1994;265:1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- Daviet L, Buckland R, Puente MD, Navazo, McGregor JL. Identification of an immunodominant functional domain on human CD36 antigen using human-mouse chimaeric proteins and homologue-replacement mutagenesis. Biochem J. 1995;305:221–224. doi: 10.1042/bj3050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes J, Clemetson KJ, Gogstad GO, McGregor J, Clezardin P, Prowse CV, Pepper DS. A radioimmunoassay for thrombospondin, used in a comparative study of thrombospondin, beta-thromboglobulin and platelet factor 4 in healthy volunteers. Thromb Res. 1983;29:569–581. doi: 10.1016/0049-3848(83)90212-8. [DOI] [PubMed] [Google Scholar]
- DiPietro LA, Nebgen DR, Polverini PJ. Downregulation of endothelial cell thrombospondin 1 enhances in vitro angiogenesis. J Vasc Res. 1994;31:178–185. doi: 10.1159/000319585. [DOI] [PubMed] [Google Scholar]
- Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- Folkman J. Clinical applications of research on angiogenesis. N Engl J Med. 1995a;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- Folkman, J. 1995b Tumor angiogenesis. In The Molecular Basis of Cancer. J. Mendelsohn, P.M. Howley, M.A. Israel, and L.A. Liotta, editors. W.B. Saunders, Philadelphia, PA. 206–232.
- Folkman J, Klagsbrun M. Angiogenic factors. Science (Wash DC) 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Frazier WA. Thrombospondins. Curr Opin Cell Biol. 1991;3:792–799. doi: 10.1016/0955-0674(91)90052-z. [DOI] [PubMed] [Google Scholar]
- Gao A-G, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the COOH-terminal domain of thrombospondin. J Biol Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- Gately S, Twardowski P, Stack MS, Patrick M, Boggio L, Cundiff DL, Schnaper HW, Madison L, Volpert O, Bouck N, et al. Human prostate carcinoma cells express enzymatic activity that converts human plasminogen to the angiogenesis inhibitor, angiostatin. Cancer Res. 1996;56:4887–4890. [PubMed] [Google Scholar]
- Gilmore AP, Romer LH. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godyna S, Liau G, Popa I, Stefansson S, Argraves WS. Identification of the low density lipoprotein receptor-related protein (LRP) as an endocytic receptor for thrombospondin-1. J Cell Biol. 1995;129:1403–1410. doi: 10.1083/jcb.129.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradishar W. An overview of clinical trials involving inhibitors of angiogenesis and their mechanism of action. J Invest New Drugs. 1997;15:49–59. doi: 10.1023/a:1005770612294. [DOI] [PubMed] [Google Scholar]
- Greenwalt DE, Lipsky RH, Ockenhouse CF, Ikeda H, Tandon NN, Jamieson GA. Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, and transfusion medicine. Blood. 1992;80:1105–1115. [PubMed] [Google Scholar]
- Guo NH, Krutzsch HC, Negre E, Zabrenetzky VS, Roberts DD. Heparin-binding peptides from the type I repeats of thrombospondin. Structural requirements for heparin binding and promotion of melanoma cell adhesion and chemotaxis. J Biol Chem. 1992;267:19349–19355. [PubMed] [Google Scholar]
- Harker LA, Marzec UM, Ginsberg MH. Thrombospondin levels in plasma, platelets and urine in normal subjects, subjects receiving heparin, and patients undergoing cardiopulmonary bypass. Thromb Haemostasis. 1983;50:22. [Google Scholar]
- Hsu SC, Volpert OV, Steck PA, Mikkelsen T, Polverini PJ, Rao S, Chou P, Bouck NP. Inhibition of angiogenesis in human glioblastomas by chromosome 10 induction of thrombospondin-1. Cancer Res. 1996;56:5684–5691. [PubMed] [Google Scholar]
- Huang M-M, Bolen JB, Barnwell JW, Shattil SJ, Brugge JS. Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc Natl Acad Sci USA. 1991;88:7844–7848. doi: 10.1073/pnas.88.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Bornstein P, Sage H. Thrombospondin exerts an antiangiogenic effect on cord formation by endothelial cells in vitro. Proc Natl Acad Sci USA. 1991;88:5026–5030. doi: 10.1073/pnas.88.11.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer N, Bettaieb A, Legrand C, Coulombel L, Vainchenker W, Edelman L, Breton-Gorius J. Developmentally regulated expression of a 78 kDa erythroblast membrane glycoprotein immunologically related to the platelet thrombospondin receptor. Biochem J. 1989;262:835–842. doi: 10.1042/bj2620835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M, D'Amore PA. Regulators of angiogenesis. Annu Rev Physiol. 1991;53:217–239. doi: 10.1146/annurev.ph.53.030191.001245. [DOI] [PubMed] [Google Scholar]
- Koch AE, Harlow LA, Haines GK, Amento EP, Unemori EN, Wong WL, Pope RM, Ferrara N. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol. 1994;152:4149–4156. [PubMed] [Google Scholar]
- Lahav J. The functions of thrombospondin and its involvement in physiology and pathology. Biochem Biophys Acta. 1993;1182:1–14. doi: 10.1016/0925-4439(93)90146-r. [DOI] [PubMed] [Google Scholar]
- Lamszus K, Joseph A, Jin L, Yao Y, Chowdhury S, Fuchs A, Polverini PJ, Goldberg ID, Rosen EM. Scatter factor binds to thrombospondin and other extracellular matrix components. Am J Pathol. 1996;149:805–819. [PMC free article] [PubMed] [Google Scholar]
- Lawler, J. 1993. The interactions of thrombospondin with integrins. In Thrombospondin. J. Lahav, editor. CRC Press, Boca Raton, FL. 275–282.
- Leung LLK, Li W-X, McGregor JL, Albrecht G, Howard RJ. CD36 peptides enhance or inhibit CD36-thrombospondin binding. J Biol Chem. 1992;267:18244–18250. [PubMed] [Google Scholar]
- Li W-X, Howard RJ, Leung LLK. Identification of SVTCG in thrombospondin as the conformation-dependent, high affinity binding site for its receptor, CD36. J Biol Chem. 1993;268:16179–16184. [PubMed] [Google Scholar]
- Mikhailenko I, Kounnas MZ, Strickland DK. Low density lipoprotein receptor-related protein/α2-macroglobulin receptor mediates the cellular internalization and degradation of thrombospondin. A process facilitated by cell-surface proteoglycans. J Biol Chem. 1995;270:9543–9549. doi: 10.1074/jbc.270.16.9543. [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Nicholson AC, Pearce SFA, Silverstein RL. Oxidized LDL binds to CD36 on human monocyte-derived macrophages and transfected cell lines. Arterioscler Thromb Vasc Biol. 1995;15:269–275. doi: 10.1161/01.atv.15.2.269. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Kashiwagi H, Yamashita S, Nakagawa T, Kostner B, Tomiyama Y, Nakata A, Ishigami M, Miyagawa J, Kameda-Takemura K. Reduced uptake of oxidized low density lipoproteins in monocyte-derived macrophages from CD36-deficient subjects. J Clin Invest. 1995;96:1859–1865. doi: 10.1172/JCI118231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse CF, Magowan C, Chulay JD. Activation of monocytes and platelets by monoclonal antibodies or malaria-infected erythrocytes binding to the CD36 surface receptor in vitro. J Clin Invest. 1989;84:468–475. doi: 10.1172/JCI114188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo P, Hundt E, Lawler J, Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparuminfected erythrocytes. Cell. 1989;58:95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Pearce SFA, Wu J, Silverstein RL. Recombinant GST/CD36 fusion proteins define a thrombospondin binding domain. J Biol Chem. 1995;270:2981–2986. doi: 10.1074/jbc.270.7.2981. [DOI] [PubMed] [Google Scholar]
- Peltzbauer P, Bender JR, Wilson J, Pober JS. Heterogeneity of dermal microvascular endothelial cell antigen expression and cytokine responsiveness in situ and in cell culture. J Immunol. 1993;151:5062–5072. [PubMed] [Google Scholar]
- Polverini PJ, Bouck NP, Rastinejad F. Assay and purification of a naturally occurring inhibitor of angiogenesis. Methods Enzymol. 1991;198:440–450. doi: 10.1016/0076-6879(91)98044-7. [DOI] [PubMed] [Google Scholar]
- Rastinejad F, Polverini PJ, Bouck NP. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell. 1989;56:345–355. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Raychaudhury A, Frazier WA, D'Amore PA. Comparison of normal and tumorigenic endothelial cells: differences in thrombospondin production and responses to transforming growth factor β. J Cell Sci. 1994;107:39–46. doi: 10.1242/jcs.107.1.39. [DOI] [PubMed] [Google Scholar]
- Ren Y, Silverstein RL, Allen J, Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J Exp Med. 1995;181:1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti A, Acton SL, Krieger M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J Biol Chem. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- Ryeom S, Silverstein RL, Scotto A, Sparrow J. Binding of anionic phospholipids to retinal pigment epithelium may be mediated by the scavenger receptor CD36. J Biol Chem. 1996a;271:20536–20539. doi: 10.1074/jbc.271.34.20536. [DOI] [PubMed] [Google Scholar]
- Ryeom S, Sparrow J, Silverstein RL. CD36 participates in the phagocytosis of rod outer segments on retinal pigment epithelium. J Cell Sci. 1996b;109:387–395. doi: 10.1242/jcs.109.2.387. [DOI] [PubMed] [Google Scholar]
- Saglio SD, Slayter HS. Use of radioimmunoassay to quantify thrombospondin. Blood. 1982;59:162–166. [PubMed] [Google Scholar]
- Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharenberg AM, Kinet J-P. The emerging field of receptor-mediated inhibitory signaling: SHP or SHIP? . Cell. 1996;87:961–964. doi: 10.1016/s0092-8674(00)81790-0. [DOI] [PubMed] [Google Scholar]
- Silverstein RL, Baird M, Lo SK, Yesner LM. Sense and antisense cDNA transfection of CD36 (Glycoprotein IV) in melanoma cells. J Biol Chem. 1992;267:16607–16612. [PubMed] [Google Scholar]
- Stellmach V, Volpert OV, Crawford SE, Lawler J, Hynes RO, Bouck N. Tumor suppressor genes and angiogenesis: the role of P53 in fibroblasts. Eur J Cancer. 1997;32A:2394–2400. doi: 10.1016/s0959-8049(96)00385-1. [DOI] [PubMed] [Google Scholar]
- Stern M, Savill J, Haslett C. Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis. Mediation by αvβ3/CD36/thrombospondin recognition mechanism and lack of phlogistic response. Am J Pathol. 1996;149:911–921. [PMC free article] [PubMed] [Google Scholar]
- Swerlick RA, Lee KH, Wick TM, Lawley TJ. Human dermal microvascular endothelial but not human umbilical vein endothelial cells express CD36 in vivo and in vitro. J Immunol. 1992;148:78–83. [PubMed] [Google Scholar]
- Tandon NN, Kralisz U, Jamieson GA. Identification of GPIV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem. 1989;264:7576–7583. [PubMed] [Google Scholar]
- Taraboletti G, Roberts D, Liotta LA, Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990;111:765–772. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboletti, G., D. Belotti, and R. Giavazzi. 1992. Thrombospondin modulates basic fibroblast growth factor activities on endothelial cells. In Angiogenesis, Key Principles. R. Steiner, P.B. Weiss, and R. Langer, editors. Birkhauser Verlag, Basel, Switzerland. 210–213. [DOI] [PubMed]
- Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolsma, S.S., M.S. Stack, and N. Bouck. 1997. Lumen formation and other angiogenic activities of cultured capillary endothelial cells are inhibited by thrombospondin-1. Microvasc. Res. In press. [DOI] [PubMed]
- Tuzinsky GP, Rothman VL, Papale M, Hamilton BK, Eyal J. Identification and characterization of a tumor cell receptor for CSVTCG, a thrombospondin adhesive domain. J Cell Biol. 1993;120:513–521. doi: 10.1083/jcb.120.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T, Guo NH, Krutsch HC, Blake DA, Hartman J, Mendelovitz S, Panet A, Roberts DD. Modulation of endothelial cell proliferation, adhesion, and motility by recombinant heparin-binding domain and synthetic peptides from the type I repeats of thrombospondin. J Cell Biochem. 1993;53:74–84. doi: 10.1002/jcb.240530109. [DOI] [PubMed] [Google Scholar]
- Volpert OV, Tolsma SS, Pellerin S, Feige J-J, Chen H, Mosher DF, Bouck N. Inhibition of angiogenesis by thrombospondin-2. Biochem Biophys Res Commun. 1995;217:326–332. doi: 10.1006/bbrc.1995.2780. [DOI] [PubMed] [Google Scholar]
- Volpert OV, Dameron KM, Bouck N. Sequential development of an angiogenic phenotype by human fibroblasts progressing to tumorigenicity. Oncogene. 1997;14:1495–1502. doi: 10.1038/sj.onc.1200977. [DOI] [PubMed] [Google Scholar]
- Weinstat-Saslow DL, Zabrentzky VS, VanHoutte K, Frazier WA, Roberts DD, Steeg PS. Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res. 1994;54:6504–6511. [PubMed] [Google Scholar]
- Williams GM, Kemp SJ, Brindle NP. Involvement of protein tyrosine kinases in regulation of endothelial cell organization by basement membrane proteins. Biochem Biophys Res Commun. 1996;229:375–380. doi: 10.1006/bbrc.1996.1813. [DOI] [PubMed] [Google Scholar]
- Yabkowitz R, Dixit VM. Human carcinoma cells bind thrombospondin through a Mr80,000/105,000 receptor. Cancer Res. 1991;51:3648–3656. [PubMed] [Google Scholar]