The heterogeneity of the endoplasmic reticulum (ER) is well established in cell biology. To a large extent, however, the differences among ER domains (such as the nuclear envelope and the rough- and smooth-surfaced cisternae) are believed to reflect their different involvement in the synthesis, transport, and local degradation of proteins. In contrast, another function of the ER, i.e., its capacity to accumulate Ca2+ and to release it quickly in response to specific signals (see reference 32), is often regarded as a property of the whole network. Yet in striated muscles, the heterogeneity of Ca2+ handling in the sarcoplasmic reticulum (SR, a specialized version of the ER) was recognized over 20 yr ago. 10 yr later, based on pioneering subcellular fractionation and immunocytochemical results, a group of laboratories first proposed that structures specialized for Ca2+ handling, the calciosomes, may also exist in nonmuscle cells (43). Shortly thereafter, a similar concept was put forth to explain the unusual (quantal) kinetics of Ca2+ release from microsomes and permeabilized cells when exposed to increasing concentrations of inositol 1,4,5-trisphosphate (IP3; reference 29). Since then, evidence of Ca2+ specializations in the ER has grown continuously, yet little effort has been made to generalize the concept and to identify mechanisms not only in biochemical and molecular but also in cell biological terms.
Here, we reconsider critically the developments in the field, focusing primarily on nonmuscle cells. We will first address the nonrandom distribution of the various ER molecular components that sustain the Ca2+ homeostatic machinery. We will then discuss the correlations between protein distribution and the complex spatio–temporal features of the Ca2+ signaling pathway, ending with a short discussion of the underlying molecular mechanisms. In contrast, we will not deal with other organelles, such as secretory granules and lysosomes, which may also work as dynamic Ca2+ storage compartments. Although repeatedly proposed, these possibilities remain open to question, so that any conclusion would be premature.
Molecular Components
Heterogeneity of Ca2+ storage and exchange within ER subcompartments depends on the nonrandom distribution of the macromolecules governing uptake, release, and binding of the cation.
Channels.
There are at least two types of channels through which Ca2+ flows from the lumen of the ER to the cytosol: IP3 and ryanodine receptors (IP3Rs and RyRs), both composed of four subunits of high molecular mass (300–500 kD). Additional channels, known only in part, will not be discussed here.
Expression of IP3Rs (three types, each including one or two alternatively spliced variants) is highly variable among cells. The quantity varies from 20,000 to 30 fmol/mg cell protein (Purkinje neurons and epididimus). Moreover, most cells express various types of IP3R subunits assembled into heterotetramers (27). Recent experiments in intact cells have revealed that this multiplicity is reflected in variations in IP3 affinity and in modulation of the release responses by cytosolic Ca2+ ([Ca2+]c). In particular, the type II IP3R was shown to be the most sensitive to IP3 and also the most susceptible to modulation. In contrast, type III appears almost insensitive to [Ca2+]c. When composed by heterotetramers, the IP3Rs tend to adopt the responses of the most sensitive/modulatable of their subunits (25).
Heterogeneous distribution of IP3Rs throughout the ER was demonstrated by both subcellular fractionation and immunocytochemistry. In the late 1980s, IP3-binding sites were reported to be enriched in rapidly sedimenting membrane fractions, caused not by plasmalemma fragments but by aggregation of specialized microsomes. In conventional microsomes IP3 binding levels were much lower (see reference 32). More recently, Western blots of microsomal subfractions obtained by velocity and density gradient centrifugation have shown that IP3Rs do partially segregate from the ER Ca2+ pumps (sarcoplasmic–endoplasmic reticulum Ca2+ ATPases [SERCAs]) and especially from the lumenal Ca2+-binding proteins (35, 41). In cells overexpressing IP3Rs (Purkinje neurons) or after cDNA transfection, the receptors appear as cylindrical structures, ∼18 nm in diameter, sticking out from the membranes, concentrated (densities up to 500/μm2, i.e., over 100-fold greater than the rest of the ER) in stacks of parallel cisternae (39, 44). In other cell types, IP3R was concentrated in vesicles and cisternae distributed either in the juxtaplasmalemma layer (smooth muscle fibers: references 7, 42; endothelia: reference 7) or in the apical area (submandibular duct cells: reference 48; pancreatic acinar cells: reference 19) of the cytoplasm. In blood neutrophils, a redistribution of IP3R-rich ER vesicles towards the phagosome takes place after exposure to opsonized particles (37). Moreover, clustering of IP3Rs into discrete puncta, corresponding to enriched ER membrane areas, has been revealed in a variety of cells whenever their [Ca2+]c was raised (47), documenting the dynamics of IP3R distribution.
Compared with the IP3Rs, the information about RyRs in nonmuscle cells is still limited. There are three subtypes of RyRs, but they are assembled as homotetramers, not heterotetramers. Type I, believed for a long time to be specific to skeletal muscle, is abundant in the brain, while types II and III are widely but not ubiquitously expressed (1, 10). Direct coupling of RyRs with the plasmalemma L-type Ca2+ channels, i.e., the interaction that drives the quick discharge of muscle SR, has been hypothesized in a few neurons showing ER cisternae parallel to the cell surface (8; see also reference 3). Elsewhere, activation of RyRs is probably triggered by rapid increases of the local [Ca2+]c (Ca2+-induced Ca2+ release). Activation of type II and possibly also of type III is modulated by a messenger, cyclic ADP ribose.
Knowledge about RyR distribution is less extensive than for IP3Rs, also because the levels of expression are lower and thus difficult to reveal, especially by immunocytochemistry. Except for the neuron superficial cisternae (8), high receptor concentrations, such as in muscle SR, have not been observed. Distribution, however, does not appear to be random, as documented by [Ca2+]c imaging in many cell types, where discharge by caffeine (the alkaloid that activates RyRs) and IP3 was shown to take place from different cisternae (12). At fine dendrites and dendritic spines of neurons, differential distribution of the two channels has been observed also by immunocytochemistry. In chicken Purkinje neuron spines, IP3Rs are numerous, whereas RyRs remain below detection (45). In contrast, RyRs are also abundant in the spines of hippocampal neurons (36), playing a considerable role in the shaping of dendritic action potentials (16).
Ca2+ Pumps.
Accumulation of Ca2+ within the stores is due to the SERCAs, the products of at least three genes (23). ER non-SERCA Ca2+ pumps have also been reported but not characterized. Among SERCAs, type 1 and 2a are expressed at high levels in skeletal and heart muscle, respectively. SERCA 2b, an alternatively spliced version of 2a, is widespread and often coexists with type 3. These pumps maintain a high free [Ca2+] within the ER/ SR lumen, in equilibrium with Ca2+ bound to the lumenal proteins (resting values in the 10−4–10−3 [free] and 10−2 [total] mol/liter, respectively: references 14, 26, 28, 31, 33).
The best information on SERCA distribution is in striated muscle, where the pump is known to occupy the SR surface, except for the junctional face occupied by RyRs. In a few nonmuscle cells, immunocytochemistry has shown a widespread distribution (20). However, subcellular fractionation has revealed dissociations of SERCAs 2a and 3 from both the IP3Rs and ER lumenal proteins (35, 41). Thus, it is likely that Ca2+ uptake is not uniformly distributed but can vary considerably among ER domains.
Lumenal Ca2+-binding Proteins and Lumenal Ca2+.
Most ER resident proteins bind Ca2+ with low affinity. They are thus able to buffer the Ca2+ concentration within the lumen, [Ca2+]l, in the 10−4–10−3 M range and to rapidly release their bound Ca2+ when [Ca2+]l drops, thus sustaining for seconds the efflux through IP3R and RyR channels. Accumulation at discrete sites could be important for the functioning of the ER Ca2+ homeostatic machinery. So far, however, this property has been observed only for calsequestrin (CSQ), the major lumenal protein of striated muscle, which is also expressed by Purkinje neurons (avian) and smooth muscle fibers (42, 44). In nonmuscle cells, the distribution of other Ca2+-binding proteins, such as calreticulin (which binds ∼50% of the Ca2+ store in the ER) and BiP, was found to be widespread by both subcellular fractionation and immunocytochemistry (31, 42). Surprisingly, however, when high-resolution maps were developed by either energy loss spectroscopy or electron probe x-ray microanalysis (applied to quick-frozen cells to preserve the in vivo localization of the cation), the distribution of total calcium within the ER lumen was found to be far from homogeneous, with calcium-rich cisternae in continuity with cisternae remaining below threshold (31; Fig. 1). In addition, long stimulations of neurons induced considerable loading of some cisternae, whereas others remained unaffected, as if they were not participating in the evoked Ca2+ fluxes (33). In terms of Ca2+ handling, therefore, the functional continuity of ER cisternae looks much less extensive than commonly believed. The apparent conflict with the results of rapid diffusion of ER lumenal proteins implies the existence of calcium segregation mechanisms, which, however, remain largely mysterious.
Figure 1.
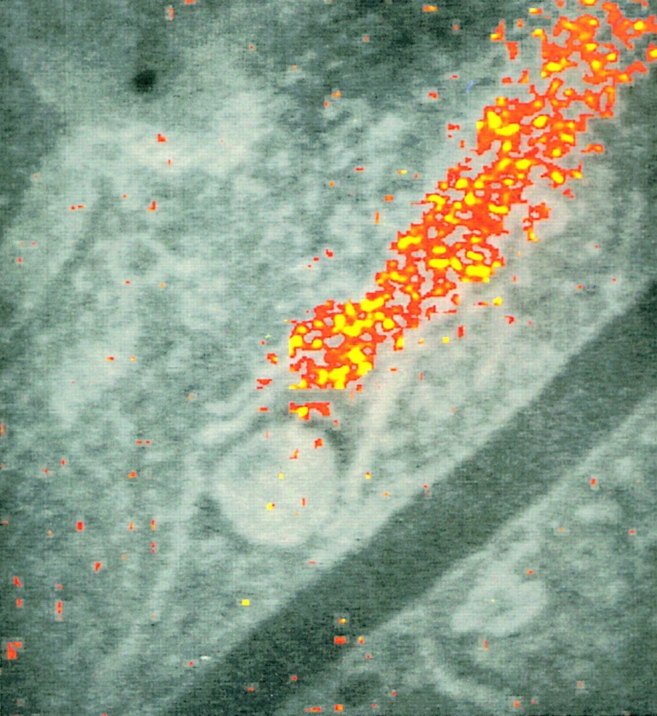
Distribution of total calcium in a PC12 pheochromocytoma cell, as revealed by electron energy loss imaging analysis. The calcium signal, expressed in false colors, is superimposed on a conventional image from a thin section of a quick frozen–freeze dried cell, including a mitochondrion and a few parallel running ER cisternae. Notice the calcium-rich ER cisternae in continuity with other cisternae where the signal remains below threshold, as it is the case with the mitochondrion and the plasmalemma. Reproduced with permission from Pezzati et al. (31).
Cell Physiology
In the past, distribution of Ca2+-governing proteins and [Ca2+]c events taking place within intact cells have often been investigated in parallel and interpreted together. Progressively, however, it has become clear that [Ca2+]c responses do not result only from the activation of individual receptors but constitute coordinate processes in which localized initiation events are followed by the activation of autoregenerative Ca2+ release (see references 2, 15, 32). The properties of these responses depend largely on the molecular properties and distribution of the proteins (especially the channels described above) governing the ER Ca2+ homeostasis.
The events that follow channel openings are governed by two general rules: the slow rate of Ca2+ diffusion throughout the cytosol and the positive modulation of most (but not all) IP3Rs and RyRs by [Ca2+]c. The first, caused by the high Ca2+ buffering of the cytosol, tends to keep [Ca2+]c increases in the proximity of the releasing organelles; the second imparts increased opening probability to adjacent channels. The resulting local [Ca2+]c increases (spikes, puffs, or sparks) can be important not only as the trigger of waves, but also by themselves. In the case of mitochondria, the affinity of Ca2+ uptake is low, in the micromolar range, and a high level is attained only in the proximity of active IP3Rs. Ca2+ accumulation by juxta-ER mitochondria can thus be rapid, resulting in strong, Ca2+-dependent activation of key dehydrogenases and consequently more efficient ATP synthesis. Mitochondria thus change their role depending on their partnership with the ER, in line with a spatial regulation of cellular energy metabolism (34). The distribution of Ca2+ spikes is also important with respect to the nucleus. Only the spikes generated in the proximity of the nucleus have high probability to invade the nucleoplasm, whereas most of those generated at some distance extinguish in the cytoplasm (22), with different effects on gene expression. Transcription of some genes is in fact controlled locally by Ca2+ rises, whereas the Ca2+ activation of others is indirect, mediated by cytoplasmic enzymes (e.g., the MAP kinases) or factors transferred to the nucleus in response to [Ca2+]c rises. Thus, Ca2+ regulation of gene expression can be defined as a space-dependent process (11). Even in the cytoplasm, differently localized [Ca2+]c events can have different, even opposite effects. In smooth muscle fibers, the sparks in the deep cytoplasm contribute to the tone, whereas those near the plasmalemma can induce relaxation via activation of Ca2+-dependent K+ channels (30).
Ca2+ waves expand from the initiating spike to the rest of the cell, inducing Ca2+ release also from ER areas of low receptor density. Rhythmic repetition of the process can result in trains of oscillations. In pancreatic and salivary acinar cells initiation of Ca2+ release takes place at the apical area, where IP3R-rich vesicles (see before) are intermingled with secretory granules. The Ca2+ signal thus generated can trigger two important responses: exocytosis of granules and generation of a wave directed towards the base (18, 40), sustained by the abundant, but IP3R-poor, ER cisternae of that area (19).
News about [Ca2+]c oscillations, which occur at frequencies around 1/min, are also exciting. Expression of single genes, mediated by the phosphorylation/dephosphorylation with ensuing nuclear-cytoplasmic migration of transcription factors, has been shown to vary specifically according to distinct Ca2+ frequency codings (4, 21). Similar mechanisms regulate the action potential–dependent gene expression in cultured neurons (5).
Mechanisms
This section is focused more on muscle because the information about nonmuscle cells is still primitive. Developmental studies of skeletal muscle fibers have revealed that clustering of L type Ca2+ channels in the plasmalemma T tubules and of RyRs in the junctional face of SR terminal cisternae occur synchronously, establishing excitation– contraction coupling units (see reference 6). In animals and cells lacking either one of the channels, some clustering of the other still takes place. The master drive of the process, therefore, cannot be one of the channels (intracellular and surface located) but may be another as yet unidentified component (6). Coordinate clustering of SR and plasmalemma channels also occurs in the heart, however, without establishment of physical coupling (38).
Aggregation of CSQ appears to be a molecular property of the lumenal Ca2+-binding protein (9, 46). Its localization within the terminal cisternae appears, in contrast, to be caused by the interaction of CSQ aggregates with membrane proteins protruding into the lumen, the triadins, and junctins (13, 17). The latter form a complex with the RyR and thus share its localization (49).
The information available for the SR might be taken as a model to orient the studies of nonmuscle cells. IP3Rs, RyRs, and SERCAs are all known to interact with multiple proteins, at both their cytosolic and lumenal domains. So far, however, interest has been focused mostly on the functional regulation of these macromolecules rather than on their heterogeneous distribution, which therefore remains unexplained.
The hypothesis that Ca2+ in nonmuscle cells is stored in distinct organelles (calciosomes), largely independently from the ER (43), has proven to be incorrect. The idea, however, has been seminal for subsequent studies demonstrating the heterogeneity of ER structures in terms of Ca2+ homeostasis, a concept now widely accepted, but still incompletely understood. In the future, rapid progress is expected in many fields, including the identification of the mechanisms by which heterogeneity is established and the integration of ER Ca2+ stores in cells physiology (see reference 24). From the information already available, we predict that the role of Ca2+ within the stores will emerge as more fundamental for the cell than presently believed, paralleling from this stand point the well-known role played by the cation within the cytosol.
Footnotes
Address all correspondence to Jacopo Meldolesi, DIBIT, Istituto Scientifico San Raffaele, Via Olgettina 58, 20132 Milano, Italy. Tel.: 39 2 2643 2770. Fax: 39 2 2643 4813. E-mail meldolesi.jacopo@hsr.it
References
- 1.Bennett DL, Cheek TR, Berridge MJ, De Smedt H, Parys JB, Missiaen L, Bootman MD. Expression and function of ryanodine receptors in nonexcitable cells. J Biol Chem. 1996;271:6356–6362. doi: 10.1074/jbc.271.11.6356. [DOI] [PubMed] [Google Scholar]
- 2.Bootman MD, Berridge MJ, Lipp P. Cooking with calcium: the recipes for composing global signals from elementary events. Cell. 1997;91:367–373. doi: 10.1016/s0092-8674(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 3.Chavis P, Fagni L, Lansman JB, Bockaert J. Functional coupling between ryanodine receptors and L-type calcium channels in neurons. Nature. 1996;382:719–722. doi: 10.1038/382719a0. [DOI] [PubMed] [Google Scholar]
- 4.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 5.Fields RD, Eshete F, Stevens B, Itoh K. Action potential-dependent regulation of gene expression: temporal specificity in Ca2+, cAMP-responsive element binding proteins, and mitogen-activated protein kinase signaling. J Neurosci. 1997;17:7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flucher BE, Franzini-Armstrong C. Formation of junctions involved in excitation-contraction coupling in skeletal and cardiac muscle. Proc Natl Acad Sci USA. 1996;93:8101–8106. doi: 10.1073/pnas.93.15.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujimoto T, Nakade S, Miyawaki A, Mikoshiba K, Ogawa K. Localization of inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae. J Cell Biol. 1992;119:1507–1513. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuichi T, Furutama D, Hakamata Y, Nakai J, Takeshima H, Mikoshiba K. Multiple types of ryanodine receptor/Ca2+release channels are differentially expressed in rabbit brain. J Neurosci. 1994;14:4794–4805. doi: 10.1523/JNEUROSCI.14-08-04794.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatti G, Podini P, Meldolesi J. Overexpression of calsequestrin in L6 myoblasts: formation of ER subdomains and their evolution into discrete vacuoles where aggregates of the protein are specifically accumulated. Mol Biol Cell. 1997;8:1712–1728. doi: 10.1091/mbc.8.9.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giannini G, Conti A, Mammarella S, Scrabogna M, Sorrentino V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol. 1995;128:893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginty DD. Calcium regulation of gene expression: isn't that spatial? . Neuron. 1997;18:183–186. doi: 10.1016/s0896-6273(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 12.Golovina VA, Blaustein MP. Spatially and functionally distinct Ca2+stores in sarcoplasmic and endoplasmic reticulum. Science. 1997;275:1643–1648. doi: 10.1126/science.275.5306.1643. [DOI] [PubMed] [Google Scholar]
- 13.Guo W, Campbell KP. Association of triadin with the ryanodine receptor and calsequestrin in the lumen of the sarcoplasmic reticulum. J Biol Chem. 1995;270:9027–9030. doi: 10.1074/jbc.270.16.9027. [DOI] [PubMed] [Google Scholar]
- 14.Hofer AM, Landolfi B, Debellis L, Pozzan T, Curci S. Free [Ca2+] dynamics measured in agonist-sensitive stores of single living intact cells: a new look at the refilling process. EMBO (Eur Mol Biol Organ) J. 1998;17:1986–1995. doi: 10.1093/emboj/17.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horne JH, Meyer T. Elementary calcium-release units induced by inositol trisphosphate. Science. 1997;276:1690–1693. doi: 10.1126/science.276.5319.1690. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs JM, Meyer T. Control of action potential-induced Ca2+ signaling in the soma of hippocampal neurons by Ca2+release from intracellular stores. J Neurosci. 1997;17:4129–4135. doi: 10.1523/JNEUROSCI.17-11-04129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones LR, Zhang L, Sanborn K, Jorgensen AO, Kelley J. Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J Biol Chem. 1995;270:30787–30796. doi: 10.1074/jbc.270.51.30787. [DOI] [PubMed] [Google Scholar]
- 18.Kasai H, Li YX, Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+waves and oscillations in exocrine pancreas. Cell. 1993;74:669–677. doi: 10.1016/0092-8674(93)90514-q. [DOI] [PubMed] [Google Scholar]
- 19.Lee MG, Xu X, Zeng W, Diaz J, Wojcikiewicz RJH, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+channels in pancreatic and salivary gland cells. J Biol Chem. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- 20.Lee MG, Xu X, Zeng W, Diaz J, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+pumps in pancreatic and salivary gland cells. J Biol Chem. 1997;272:15771–15776. doi: 10.1074/jbc.272.25.15771. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell permeant caged InsP3 ester shows that Ca2+spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 22.Lipp P, Thomas D, Berridge MJ, Bootman MD. Nuclear calcium signalling by individual cytoplasmic calcium puffs. EMBO (Eur Mol Biol Organ) J. 1997;16:7166–7173. doi: 10.1093/emboj/16.23.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLennan DH, Rice WJ, Green NM. The mechanism of Ca2+ transport by sarco(endo)plasmic reticulum Ca2+-ATPases. J Biol Chem. 1997;272:28815–28818. doi: 10.1074/jbc.272.46.28815. [DOI] [PubMed] [Google Scholar]
- 24.Meldolesi J, Pozzan T. The ER Ca2+store: a view from the lumen. Trends Biochem Sci. 1998;23:10–15. doi: 10.1016/s0968-0004(97)01143-2. [DOI] [PubMed] [Google Scholar]
- 25.Miyakawa, T., A. Maeda, T. Yamazawa, K. Hirose, T. Kurosaki, and M. Iino. 1998. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO (Eur. Mol. Biol. Organ.) J. In press. [DOI] [PMC free article] [PubMed]
- 26.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 27.Monkawa T, Miyawaki A, Sugiyama T, Yoneshima H, Yamamoto HM, Furuichi T, Saruta T, Hasegawa M, Mikoshiba K. Heterotetratrimeric complex formation of inositol 1,4,5-trisphosphate receptor subunits. J Biol Chem. 1995;270:14700–14704. doi: 10.1074/jbc.270.24.14700. [DOI] [PubMed] [Google Scholar]
- 28.Montero M, Barrero MJ, Alvarez J. [Ca2+] microdomains control agonist-induced Ca2+release in intact HeLa cells. FASEB (Fed Am Soc Exp Biol) J. 1997;11:881–885. doi: 10.1096/fasebj.11.11.9285486. [DOI] [PubMed] [Google Scholar]
- 29.Muallem S, Pandol SJ, Beeker TG. Hormone-evoked calcium release from intracellular stores is a quantal process. J Biol Chem. 1989;246:205–212. [PubMed] [Google Scholar]
- 30.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 31.Pezzati R, Bossi M, Podini P, Meldolesi J, Grohovaz F. High resolution calcium mapping of the ER-Golgi-exocytic membrane system. Electron energy loss imaging analysis of quick frozen-freeze dried PC12 cells. Mol Biol Cell. 1997;8:1501–1512. doi: 10.1091/mbc.8.8.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular Ca2+stores. Physiol Rev. 1994;74:595–637. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- 33.Pozzo-Miller LD, Pivovarova NB, Leapman RD, Buchanana RA, Reese TS, Andrews SB. Activity-dependent calcium sequestration on dendrites of hippocampal neurons in brain slices. J Neurosci. 1997;17:8729–8738. doi: 10.1523/JNEUROSCI.17-22-08729.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 35.Rooney E, Meldolesi J. The endoplasmic reticulum in PC12 cells. Evidence for a mosaic of domains differently specialized in Ca2+handling. J Biol Chem. 1996;271:29304–29311. doi: 10.1074/jbc.271.46.29304. [DOI] [PubMed] [Google Scholar]
- 36.Sharp AH, McPherson PS, Dawson TM, Aoki C, Campbell KP, Snyder SH. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+release channels in rat brain. J Neurosci. 1993;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stendhal O, Krause K-H, Krischer J, Jerström P, Theler J-M, Clark RA, Carpentier J-L, Lew DP. Redistribution of intracellular Ca2+stores during phagocytosis in human neutrophils. Science. 1994;265:1439–1441. doi: 10.1126/science.8073285. [DOI] [PubMed] [Google Scholar]
- 38.Sun X-H, Protasi F, Takahashi M, Takeshima H, Ferguson DG, Franzini-Armstrong C. Molecular architecture of membranes involved in excitation–contraction coupling of cardiac muscle. J Cell Biol. 1995;129:659–671. doi: 10.1083/jcb.129.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takei K, Mignery GA, Mugnaini E, Südhof TC, De Camilli P. Inositol 1,4,5-trisphosphate receptor causes formation of ER cisternal stacks in transfected fibroblasts and in cerebellar Purkinje cells. Neuron. 1994;12:327–342. doi: 10.1016/0896-6273(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 40.Thorn P, Moreton R, Berridge M. Multiple, coordinated Ca2+-release events underlie the inositol trisphosphate-induced local Ca2+spikes in mouse pancreatic acinar cells. EMBO (Eur Mol Biol Organ) J. 1996;15:999–1003. [PMC free article] [PubMed] [Google Scholar]
- 41.Vanlingen S, Parys JB, Missiaen L, De Smedt H, Wuytack F, Casteels R. Distribution of inositol 1,4,5-trisphosphate receptor isoforms, SERCA isoforms and Ca2+binding proteins in RBL-2H3 rat basophilic leukemia cells. Cell Calc. 1997;22:475–486. doi: 10.1016/s0143-4160(97)90075-0. [DOI] [PubMed] [Google Scholar]
- 42.Villa A, Podini P, Panzeri MC, Soling HD, Volpe P, Meldolesi J. The endoplasmic–sarcoplasmic reticulum of smooth muscle: immunocytochemistry of vas deferens fibers reveals specialized subcompartments differently equipped for the control of Ca2+homeostasis. J Cell Biol. 1993;121:1041–1051. doi: 10.1083/jcb.121.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volpe P, Krause KH, Hashimoto S, Zorzato F, Pozzan T, Meldolesi J, Lew DP. “Calciosome,” a cytoplasmic organelle: the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? . Proc Natl Acad Sci USA. 1988;85:1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volpe P, Villa A, Damiani E, Sharp AH, Podini P, Snyder SH, Meldolesi J. Heterogeneity of microsomal Ca2+stores in chicken Purkinje neurons. EMBO (Eur Mol Biol Organ) J. 1991;10:3183–3189. doi: 10.1002/j.1460-2075.1991.tb04880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walton PD, Airey JA, Sutko JL, Beck CF, Mignery GA, Südhof TC, Deerinck TJ, Ellisman MH. Ryanodine and inositol trisphosphate receptors coexist in avian cerebellar Purkinje neurons. J Cell Biol. 1991;113:1145–1157. doi: 10.1083/jcb.113.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S, Trumble WR, Liao H, Wesson CR, Dunker AK, Kang CH. Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat Struct Biol. 1998;5:476–483. doi: 10.1038/nsb0698-476. [DOI] [PubMed] [Google Scholar]
- 47.Wilson BS, Pfeiffer JR, Smith AJ, Oliver JM, Oberdorf JA, Wojcikiewicz RJH. Calcium-dependent clustering of inositol 1,4,5-trisphosphate receptors. Mol Biol Cell. 1998;9:1465–1478. doi: 10.1091/mbc.9.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto-Hino M, Miyawaki A, Segawa A, Adachi E, Yamashina S, Fujimoto S, Sugiyama T, Furuichi T, Hasegawa T, Mikoshiba K. Apical vesicles bearing inositol 1,4,5-trisphosphate receptors in the Ca2+initiation site of ductal epithelium of submandibular gland. J Cell Biol. 1998;141:135–142. doi: 10.1083/jcb.141.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. J Biol Chem. 1997;272:23389–23397. doi: 10.1074/jbc.272.37.23389. [DOI] [PubMed] [Google Scholar]


