Abstract
The Amt/Mep ammonium channels are trimers in which each monomer contains a long, narrow, hydrophobic pore. Whether the substrate conducted by these pores is NH3 or NH4+ remains controversial. Substitution of leucine for the highly conserved tryptophan 148 residue at the external opening to Escherichia coli AmtB pores allowed us to address this issue. A strain carrying AmtBW148L accumulates much larger amounts of both [14C]methylammonium and [14C]methylglutamine in a washed cell assay than a strain carrying wild-type AmtB. Accumulation of methylammonium occurs within seconds and appears to reflect channel conductance, whereas accumulation of methylglutamine, which depends on the ATP-dependent activity of glutamine synthetase, increases for many minutes. Concentration of methylammonium was most easily studied in strains that lack glutamine synthetase. It is eliminated by the protonophore carbonyl cyanide m-chlorophenyl hydrazone and is ≈10-fold higher in the strain carrying AmtBW148L than wild-type AmtB. The results indicate that AmtB allows accumulation of CH3NH3+ ion in response to the electrical potential across the membrane and that the rate of flux through AmtBW148L is ≈10 times faster than through wild-type AmtB. We infer that both mutant and wild-type proteins also carry NH4+. Contrary to our previous views, we assess that E. coli AmtB does not differ from plant Amt proteins in this regard; both carry ions. We address the role of W148 in decreasing the activity and increasing the selectivity of AmtB and the implications of our findings with respect to the function of Rh proteins, the only known homologues of Amt/Mep proteins.
Keywords: ammonium transport, AmtB channel, carbon dioxide, enteric bacteria, Rh protein
Physiological and recent structural evidence indicates that Amt (ammonium transport) proteins, which are also called Mep (methylammonium permease), are channels for some form of ammonium (used to designate both NH4+ and NH3) and its analogue methylammonium (CH3NH3+ and CH3NH2) (1–6). Amt/Mep channels contain very long, narrow, hydrophobic pores. Several groups have provided evidence that charged NH4+ is the substrate for the Amt proteins of vascular plants and that the electrical potential across the membrane can be used to concentrate it (7–9). Our laboratory has taken the opposite view for the Escherichia coli AmtB protein, i.e., that its substrate is NH3 (10). Much of the controversy stems from difficulties inherent in the traditional [14C]methylammonium uptake assays for Amt/Mep function in microbes, which depend on ATP-dependent coupling reactions. In washed cells of enteric bacteria, accumulation of label from [14C]methylammonium, which occurs over many minutes, is coupled to ATP-dependent synthesis of [14C]methylglutamine by glutamine synthetase (GS) (5, 10, 11). Under these circumstances, it is difficult to establish a role for the electrochemical potential gradient across the membrane separate from its role in ATP synthesis. This is also the case in Saccharomyces cerevisiae, where accumulation of unmodified [14C]methylammonium is coupled to the function of the vacuolar ATPase (10, 12).
Amt/Mep proteins are trimers (13) in which each monomer contains a pore. The very hydrophobic pores admit water poorly and appear suited to conduct NH3 or CH3NH2. This fact and studies of the function of E. coli AmtB in proteoliposomes led Khademi et al. (2) to propose independently that Amt/Mep proteins were NH3 gas channels. Several molecular dynamic simulations of the behavior of NH4+ and NH3 in the pores appeared to confirm this, although efforts to repeat the assays in proteoliposomes did not (6).
Khademi et al. (2) noted that the highly conserved aromatic residue W148 (numbering for E. coli AmtB) was part of a collar at the external entry to Amt pores. They postulated that a primary function of W148 and the other aromatic residues forming the collar was to recruit the NH4+ ion by means of cation attraction to their π electrons. They also postulated that NH4+ recruitment was essential to channel conductance of NH3.
Having isolated strains in which W148 was changed to L by using a selection described briefly in Results, we became interested in whether W148, in particular, was required for activity of AmtB. As discussed below, our studies indicated that changing W148 to a smaller nonaromatic residue greatly increased rather than decreased channel function. They further indicated that the modified AmtB protein allowed rapid accumulation of CH3NH3+ ion in response to the membrane potential. In agreement with the findings of others (8, 9), we now conclude that CH3NH3+ and NH4+ are the substrates for Amt/Mep proteins and that Amt/Mep channels can concentrate these ions in the cell interior.
Results
We have studied a strain of E. coli in which the highly conserved W148 residue of AmtB was changed to L. We found this change repeatedly in a selection for suppression of the growth defect at low NH3 (0.5 mM total ammonium = NH4+ + NH3 at pH 5.5) caused by AmtBL416A (R.N.F., K.-S.K., C.Y., W.B.I., and S.K., unpublished work). W148L restored both good growth at low NH3 and some [14C]methylammonium uptake to a strain with L416A.
A strain carrying AmtB with the W148L substitution alone (i.e., in the absence of L416A) grew as well at low NH3 as a congenic wild-type strain (Fig. 1). By contrast, a strain lacking AmtB had a pronounced growth defect under these conditions (10, 11).
Fig. 1.
A strain carrying AmtBW148L grows normally at low NH3. Light scattering as a function of time is plotted for a strain carrying AmtBW148L [NCM4530 (amtBW148L tesB::Kan)], a strain carrying an amtB null allele [NCM4310 (amtB::Spc)], and the parental strain for both, NCM3722. Strains were grown at low pH and low ammonium as described in Materials and Methods. The doubling time for strains NCM4530 and NCM3722 was 50 min. The initial doubling time of NCM4310 was 200 min.
The amtBW148L strain took up label from [14C]methylammonium (initial external concentration of 6 μM; pKa of ≈10.7) approximately five times faster than a wild-type strain (Fig. 2). If the cell suspension of the amtBW148L strain was diluted between 2- and 8-fold so that there were fewer cells present during the assay, and they took up less of the total [14C]methylammonium provided, the normalized rate of uptake appeared to be as much as 10 times faster than that of wild-type [≈500 pmol/(ml·OD600·min) versus 50]. Dilution had no effect on the rate of uptake of 14C label by the wild-type strain. Western blot analysis indicated that the AmtBW148L protein was present in the same or lower amounts as wild-type AmtB [supporting information (SI) Fig. 8]. Its amount was not elevated, and hence higher activity was not due to increased protein.
Fig. 2.
The amtBW148L mutation greatly increases uptake of label from [14C]methylammonium in a washed-cell assay. Accumulation of label from 14CH3NH3Cl (initial external concentration of 6 μM) is plotted as a function of time. For a strain carrying AmtBW148L (NCM4530) accumulation was measured at our standard OD600 value of ≈1.0 and was also measured at 1/2, 1/4, and 1/8 this value and normalized to OD 1. For parental strain NCM3722, accumulation was measured at an OD600 of ≈1.0 and at 1/2 this value.
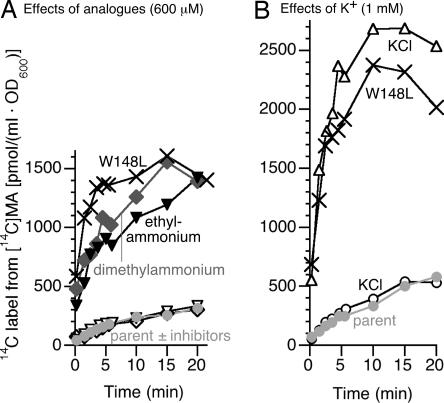
Accumulation of label from [14C]methylammonium by the amtBW148L strain was noticeably inhibited by the larger ammonium analogues dimethylammonium and ethylammonium (600 μM; Fig. 3A), whereas these analogues had no inhibitory effect on accumulation of label by an amtB+ strain. By contrast, K+ at 1 mM or 10 mM did not inhibit [14C]methylammonium uptake (Fig. 3B and data not shown) and, in fact, stimulated slightly at 1 mM.
Fig. 3.
Methylammonium uptake by AmtBW148L is inhibited by larger NH4+ analogues, but not by K+. Accumulation of label from 14CH3NH3Cl (initial external concentration of 6 μM) is plotted as a function of time for strains NCM4530 (amtBW148L tesB::Kan) and NCM3722. (A) Accumulation was determined in the absence of larger analogues and in the presence of 600 μM dimethylamine hydrochloride (diamonds) or ethylamine hydrochloride (inverted triangles). (B) Accumulation was determined in the absence of K+ and in the presence of 1 mM KCl.
To determine whether accumulation of label from [14C]methylammonium by the amtBW148L strain depended on the function of GS, we introduced into this strain two nonpolar mutations in the glnA gene, which codes for GS. One was a point mutation [glnA1859; (14)] that changed S319 to F, and the other was a complete deletion (15). Western blotting indicated that both of the amtBW148L glnA double-mutant strains synthesized AmtB (data not shown), and they rapidly accumulated label from [14C]methylammonium to levels 20–30% of those accumulated by the parental amtBW148L strain (undiluted) (Fig. 4 versus Figs. 2 and 3). Maximal levels of accumulation of label, which were comparable with or higher than those ever seen in a wild-type strain (i.e., amtB+ glnA+; Figs. 2 and 3), were reached within 30 seconds. When the same glnA point mutation and deletion were introduced into an amtB+ strain, there was a small but detectable residual accumulation of label from [14C]methylammonium (Fig. 5 and similar data not shown). Again, accumulation occurred very rapidly. We had failed to note this small accumulation in amtB+ glnA strains previously, partly because our efforts to increase its detection by increasing cell density reduced it to background (R.N.F. and S.K., unpublished work).
Fig. 4.
An amtBW148L ΔglnA double mutant strain accumulates [14C]methylammonium in response to the proton motive force. Strain NCM4598 (amtBW148L ΔglnA) was preloaded with 14C label by exposing it to [14C]methylammonium (6 μM) (as in Fig. 3). (A) At 10 min, unlabeled methylammonium (MA) was added to a final concentration of 1 mM. The control in which no cold methylammonium was added was run in a separate experiment. [14C]methylammonium uptake was also determined for cells in which glucose was omitted during the 15 min preincubation but included during harvesting and washing of cells. (B) At 10 min, the protonophore CCCP was added to the experimental sample to a final concentration of 100 μM. In this experiment, cells were aerated by shaking.
Fig. 5.
A ΔglnA strain accumulates [14C]methylammonium in response to the proton motive force. Accumulation of label from [14C]methylammonium was determined for strains NCM4353 (ΔglnA) and NCM4596 (ΔglnA ΔamtB). It was also determined for NCM4353 when CCCP (100 μM final concentration) was added to the preincubation buffer 5 min before the start of the assay. Note the difference in scale from Figs. 3 and 4.
Based on chromatographic characterization, the 14C-labeled material accumulated in washed cells of an amtBW148L glnA double-mutant strain was entirely [14C]methylammonium (Fig. 6A), as was the case for the small amount of label accumulated in washed cells of an amtB+ glnA strain (Fig. 6C). The 14C-labeled material accumulated by the amtBW148L glnA+ strain was partly [14C]methylglutamine and partly unmodified [14C]methylammonium (Fig. 6B and Table 1). Although the labeled material accumulated in amtB+ strains was largely [14C]methylglutamine, there was a small amount of unmodified [14C]methylammonium [which we failed to note previously (11); Fig. 6D]. In agreement with chromatographic characterization of labeled products (Fig. 6), addition of 1 mM or 10 mM unlabeled methylammonium to an amtBW148L glnA strain at 10 min after the start of a [14C]methylammonium uptake assay resulted in essentially complete loss of 14C label within 1 min (Fig. 4A and similar data not shown). By contrast, addition of unlabeled methylammonium at 10 min caused little loss of 14C label from the amtBW148L strain over the ensuing 10 min (SI Fig. 9). We calculate that the amtBW148L glnA strain concentrated [14C]methylammonium ≈100-fold over the 6 μM provided externally, whereas the amtB+ glnA strain concentrated it at most 5- to 10-fold (ref. 5; see Materials and Methods for conversion factors and limits of detection).
Fig. 6.
Characterization of products accumulated from [14C]methylammonium by thin-layer chromatography. Cells were incubated with [14C]methylammonium (6 μM; 14 cpm/pmol for A, B, and D and 45 cpm/pmol for C) for the times indicated. Labeled products were extracted and characterized by thin-layer chromatography as described in Materials and Methods. MA and MG are the methylammonium and methylglutamine standards (shown in A and B), respectively. The position of the MA standard is indicated by an arrow. For the samples in A and B, ≈800 cpm (≈57 pmol of product) was loaded in each lane to facilitate estimation of the proportions of [14C]methylammonium and [14C]methylglutamine present at different times (Table 1). For the samples in C and D, equal volumes were loaded. In C, they contained between 300 and 100 cpm and for D between 500 and 1,100 counts per minute (increasing with time). (A) NCM4588 (amtBW148L glnA1859). (B) NCM4530 (amtBW148L). (C) NCM4353 (ΔglnA). (D) NCM4236 (tesB::Kan; wild-type congenic with the amtBW148L strains).
Table 1.
Calculated amounts of 14C-labeled products accumulated by NCM4530 (amtBW148L) and NCM4236 (congenic wild-type)
| Time point, min | W148L |
Congenic wild-type |
||||
|---|---|---|---|---|---|---|
| Total 14C label, pmol/(ml·OD600) | [14C]MA, % | [14C]MG, % | Total 14C label, pmol/(ml·OD600) | [14C]MA, % | [14C]MG, % | |
| 2 | 1,520 | 65 | 35 | 210 | 6 | 94 |
| 4 | 1,880 | 69 | 31 | 310 | 7 | 93 |
| 6 | 2,200 | 33 | 67 | 360 | 1 | 99 |
| 11 | 2,550 | 35 | 65 | 450 | 5 | 95 |
| 21 | 2,850 | 29 | 71 | 600 | 5 | 95 |
| 41 | 2,750 | 18 | 82 | |||
Total labeled product was determined as in Figs. 2 and 3. Percentages of [14C]methylammonium ([14C]MA) and [14C]methylglutamine ([14C]MG) were approximated from the chromatograms of Fig. 6 by using ImageQuant analysis of PhosphorImager scans. Levels of 5% [14C]methylammonium for NCM4236, which correspond to 30 pmol/(ml·OD600) at the 21-min time point, are at our limit of detection. They correspond to ≈5-fold concentration of the external [14C]methylammonium provided (6 μ M).
Accumulation of [14C]methylammonium by washed cells of amtBW148L glnA depended on an energy source (Fig. 4A) and occurred to higher levels if glucose concentrations lower than our standard 0.2% were used and cultures were shaken to aerate them (data not shown). When cultures were shaken, label was apparently retained for longer (Fig. 4B and similar data not shown). To assess whether accumulation depended on the proton motive force, we used the proton ionophore carbonyl cyanide m-chlorophenyl hydrazone (CCCP) to dissipate it (ref. 16, p. 211). Preincubation with CCCP at 100 μM for 5 min before the start of an assay prevented accumulation of [14C]methylammonium by both the amtBW148L glnA strain (SI Fig. 10A) and the amtBW148L glnA+ strain (SI Fig. 10B). Lower concentrations of CCCP had a smaller effect. Addition of CCCP to 100 μM at 10 min after the start of a methylammonium uptake assay caused essentially complete loss of 14C label from an amtBW148L glnA strain within 1 min (Fig. 4B). Preincubation with CCCP at 100 μM also decreased accumulation of the small amount of label taken up by an amtB+ ΔglnA strain to background levels (Fig. 5). Introduction of a ΔamtB mutation into the ΔglnA strain likewise prevented the accumulation of label. Both indicated that the low levels of accumulation in the ΔglnA strain were meaningful.
Discussion
A strain with the AmtBW148L substitution grows optimally at low NH3 (Fig. 1) and shows enhanced uptake of label from [14C] methylammonium, a larger ammonium analogue (Figs. 2 and 3). [14C]methylammonium uptake has become partially sensitive to the still larger analogues dimethylammonium and ethylammonium but is not sensitive to K+ (Fig. 3). Most striking, the AmtBW148L substitution appears to allow much greater accumulation of CH3NH3+ ion (10- to 20-fold) than the wild-type protein. Accumulation is easily studied in the absence of GS (Fig. 4 vs. Fig. 5 and ref. 5). It occurs within 30 seconds and is dissipated by a protonophore, indicating that it depends on the electrochemical potential gradient across the membrane [presumably the electrical component ΔΨ (ref. 16, p. 71)] Strains with the mutant protein could not be concentrating CH3NH2, because this is a neutral species to which the membrane is permeable (17). Increased accumulation of CH3NH3+ ion by a strain that has GS and carries AmtBW148L results in a comparably increased rate of synthesis of methylglutamine over the ensuing minutes, providing evidence that the ionic species is the normal substrate for AmtB. [Both the in vitro and apparent in vivo Km values of GS for methylammonium are much higher than the 6 μM we have used (5, 18).] The importance of carrying an ion is confirmed by the finding that strains lacking AmtB synthesize essentially no methylglutamine under our experimental conditions (5, 10, 11), despite the fact that neutral CH3NH2 presumably crosses the membrane rapidly in an unmediated manner (17). Although we have no direct proof, we infer that both AmtBW148L and wild-type AmtB also conduct the NH4+ ion. We know that the W148L substitution increases flux of some form of ammonium because it restores growth at low NH3 in a strain with the AmtBL416A substitution (see Results; W.B.I. and S.K., unpublished work).
The AmtBW148L protein conducts CH3NH3+ ion at least 10 times faster than the wild-type protein. As discussed above, the internal concentration of CH3NH3+ ion in an amtBW148L glnA strain, which lacks GS, is 10-fold higher than that in an amtB+ glnA strain. Hence, unmediated outward diffusion of CH3NH2 must be 10 times faster in the strain carrying AmtBW148L (Fick's first law). At steady state, the rate of inward flux of the ion must be equal to this. The calculation gives a minimum estimate of the increase in the rate of flux through AmtBW148L because it assumes that the electrochemical potential gradient across the membrane is the same in strains with the mutant and wild-type proteins. Nonetheless, the degree of concentration of CH3NH3+ by strains that have AmtBW148L appears to approach the theoretical limit of ≈100-fold [ΔΨ = ≈−120 mV (ref. 16, p. 71)].
Taken together, our results indicate that the highly conserved W148 residue is not required for recruitment of the NH4+ ion or the CH3NH3+ ion by Amt/Mep proteins (growth of the amtBW148L strain is normal at low NH3, at least in batch culture at low pH, and uptake of [14C]methylammonium is increased). Rather, a primary role of W148 appears to be to restrict conductance of CH3NH3+ and larger NH4+ analogues. The genetic selection used to isolate the W148L substitution provides preliminary evidence that W148 also restricts conductance of NH4+. Modeling the W148L substitution indicates that it enlarges the opening to the AmtB pore (Fig. 7). Whether this is actually the case or is the basis for increased activity and decreased selectivity of the mutant channel remains to be determined.
Fig. 7.
The W148L substitution appears to enlarge the entry to the AmtB pore. Models of the periplasmic (exterior) face of the AmtB pore were created by using PyMol (19) from Protein Data Bank entry 2NUU deposited by Conroy et al. (20). They are shown in an orientation close to that originally used by Khademi et al. (2). A shows residues surrounding the opening to the pore in wild-type AmtB, and B shows predictions for the mutant W148L protein. W148 (A) and L148 (B) are in green and are marked with asterisks. Arrows have been placed over the conserved F107 residue (also green), and point toward pore openings. Because of its proximity to F107, W148 blocks entry to the AmtB pore. The pore opening appears larger in the W148L mutant, and H168 and H318, which lie deep in the channel and are critical for ammonium conductance (2, 21), are visible. They are indicated in green stick form. The gray residue at the top right of A and B is F103, and the black residue at the bottom right is S219.
As there has been controversy over the substrate for Amt/Mep proteins, there has been ongoing dispute over the substrate for Rh proteins, their only known homologues (6, 8–10). Our laboratory has previously proposed that Amt/Mep and Rh proteins are biological gas channels for NH3 and CO2, respectively (10). These gases have in common that they are readily hydrated. As discussed above, Amt/Mep proteins appear to conduct and concentrate NH4+ and CH3NH3+ ions, although in a restricted way. The NH4+ ion is the hydrated form of NH3 gas [NH4OH is undetectable (22, 23)]. Evidence is accumulating that the substrate for Rh proteins is some form of carbon dioxide (10, 24–27). By analogy, we postulate that Rh proteins conduct H2CO3, the hydrated form of CO2 (equivalent to H+ + HCO3−). In this case, a pH gradient, but not an electrical gradient, could drive flux. Our revised hypothesis is that Amt/Mep and Rh proteins have in common that they carry hydrated gases, in one case an ion and in the other a neutral species. Evidence in oocytes indicates that Rh proteins do conduct a neutral species (8), although the experiments were all done with ammonium and methylammonium. By contrast, similar evidence indicates that the LeAMT1;1 protein from tomato conducts NH4+ and CH3NH3+ ions. Unfortunately, E. coli AmtB did not work in oocytes, nor did the equivalent of the W148L substitution (W178L) in LeAMT1;1 (28).
Materials and Methods
Strains, Plasmids, and Growth Conditions.
To separate the W148L substitution in amtB from the L416A substitution, the W148L substitution was introduced by site-directed mutagenesis into the plasmid pGEM-T Easy (Promega, Madison, WI) carrying the wild-type glnK-amtB region and an adjacent tesB::Kan insertion. The mutagenized fragment was amplified, gel purified, and electroporated into strain NCM4172 (amtB::Spc) (11) carrying pKD46 (λ red helper plasmid). The desired transformant, NCM4530 (amtBW148L tesB::Kan) was identified among Kanr Spcs transformants by sequencing. Strain NCM4236 (tesB::Kan) is congenic to NCM4530. NCM3722 (29) is the appropriate parental strain because the amtB::Spc mutation in NCM4172 is in this background. To construct strain NCM4353 (ΔglnA), strain NCM3722 was transduced to kanamycin resistance with P1 phage grown on strain JWK3841-1 (glnA::Kan) from the Keio collection and the kanamycin insertion was then removed by site-specific recombination (14). Strain NCM4588 (amtBW148L tesB::Kan glnA1859 zih102::Tn10) was constructed by transducing strain NCM4530 to tetracycline resistance with phage grown on strain NCM1649 (glnA1859 zih102::Tn10) and screening for glutamine auxotrophy. Strain NCM4596 (ΔglnA ΔamtB) was constructed by transducing strain NCM4353 to kanamycin resistance with phage grown on Keio strain JWK0441-1 (amtB::Kan) to yield strain NCM4595 and then excising the amtB lesion by site-specific recombination. Strain NCM4598 (amtBW148L tesB::Kan ΔglnA) was constructed by transducing NCM4596 to kanamycin resistance with phage grown on strain NCM4530.
Growth at low NH3 refers to growth in Neidhardt's medium (30) with 80 mM Mes buffer (pH 5.5) instead of Mops buffer and 0.5 mM NH4Cl as nitrogen source. The carbon source was glucose (0.1%). The pKa of ammonium is ≈9.25, and hence the initial concentration of NH3 under these conditions is 100 nM. For MA uptake assays and Western analysis, cells were grown in N−C− medium, which has a pH of 7.0, with 3 mM glutamine as nitrogen source, a nitrogen-limiting condition (11). The carbon source was glucose (0.4%). For Western blot analysis, they were also grown with both 3 mM glutamine and 10 mM NH4Cl, a nitrogen-excess condition.
Methylammonium-Uptake Assays.
Cultures were grown overnight as described above. They were inoculated at 1:1,000 from precultures grown on N−C− medium containing 5 mM NH4Cl or 5 mM NH4Cl plus 3 mM glutamine as nitrogen sources. Cultures were harvested and held on ice until used. Unless noted otherwise, the OD600 of the cell suspension used for assay was ≈1.0, and the suspensions were not actively aerated. The buffer for harvesting, preincubation, assay, and washing contained 50 mM Hepes (pH 7), 72 mM NaCl, and 0.2% glucose. Omission of glucose or inclusion of inhibitors during the 15-min preincubation is noted. Assays were performed at 37°C essentially as described (11), except that the wash buffer was at room temperature (≈22°C). The amount of label retained on filters depended on the temperature of the wash buffer. It was lowest at 37°C, where filtration was fastest, and vice versa at 4°C (K.-S.K. and S.K., unpublished work). Whereas relative values for strains carrying AmtBW148L and wild-type AmtB are meaningful, the significance of absolute values is not clear, particularly for glnA strains, which accumulate only [14C]methylammonium. The meaning of absolute values depends on the degree to which the assay mimics the behavior of cell suspensions before they are filtered. The same is true of filtration assays for studying the binding of small molecules to proteins (31).
14C-labeled products in cells were analyzed as described (11). Labeled products were extracted from cells in 70% ethanol containing 0.05% SDS and were characterized by thin-layer chromatography in isopropanol water (3:1) in comparison with [14C]methylammonium and [14C]methylglutamine standards (32).
The approximate degree of concentration of [14C]methylammonium by glnA strains was calculated by using the conversion factors 0.45 mg of dry weight/(ml·OD600) and 2 μl of internal volume per milligram of dry weight (33). Our limit of detection is ≈25 pmol/(ml·OD600), which corresponds to a degree of concentration of ≈5-fold. Rates of uptake for glnA+ strains were not constant over the first few minutes of the assay and hence can only be approximated. Thereafter, they declined due to [14C]methylglutamine excretion (W.B.I. and S.K., unpublished work).
Western Blot Analysis.
Frozen cell pellets were suspended in a Tris extraction buffer (Novagen, San Diego, CA) containing a nondenaturing detergent, lysozyme, and DNase. After addition of gel sample buffer and DTT (final concentrations 2% LDS and 50 mM DTT), proteins were separated by 4–12% SDS/PAGE and transferred to nitrocellulose membranes (Whatman, Schleicher & Schuell). Membranes were immunoblotted with rabbit polyclonal antibodies directed against C-terminally His-tagged AmtB (1), and these were detected with alkaline phosphatase-conjugated secondary antibody (Invitrogen, Carlsbad, CA) and the chromogenic substrates 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (Bio-Rad). AmtB-His6 was a kind gift from Helen Zgurskaya (University of Oklahoma, Norman, OK), and antibodies were produced by Invitrogen by using standard protocols. They were preadsorbed with a cell extract of the amtB null strain NCM4310 before use. Prestained molecular weight markers (10–200 kDa) were from Invitrogen.
Supplementary Material
Acknowledgments
We thank Laszlo Csonka, Franklin Harold, Terence Hwa, Boris Magasanik, Hiroshi Nikaido, David Wemmer, Dalai Yan, and Helen Zgurskaya for critical discussions and comments on the manuscript and the National BioResource Project of the National Institute of Genetics, Japan, for E. coli Keio strains JWK3841–1 (glnA::Kan) and JWK0441–1 (amtB::Kan). This work was funded by National Institutes of Health Grant GM38361 (to S.K.).
Abbreviations
- CCCP
carbonyl cyanide m-chlorophenyl hydrazone
- GS
glutamine synthetase.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709267104/DC1.
References
- 1.Soupene E, Chu T, Corbin RW, Hunt DF, Kustu S. J Bacteriol. 2002;184:3396–3400. doi: 10.1128/JB.184.12.3396-3400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khademi S, O'Connell J, III, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM. Science. 2004;305:1587–1594. doi: 10.1126/science.1101952. [DOI] [PubMed] [Google Scholar]
- 3.Zheng L, Kostrewa D, Berneche S, Winkler FK, Li XD. Proc Natl Acad Sci USA. 2004;101:17090–17095. doi: 10.1073/pnas.0406475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade SL, Dickmanns A, Ficner R, Einsle O. Proc Natl Acad Sci USA. 2005;102:14994–14999. doi: 10.1073/pnas.0506254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Javelle A, Thomas G, Marini AM, Kramer R, Merrick M. Biochem J. 2005;390:215–222. doi: 10.1042/BJ20042094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javelle A, Lupo D, Li XD, Merrick M, Chami M, Ripoche P, Winkler FK. J Struct Biol. 2007;158:472–481. doi: 10.1016/S1047-8477(07)00165-7. [DOI] [PubMed] [Google Scholar]
- 7.von Wirén N, Gazzarrini S, Gojon A, Frommer WB. Curr Opin Plant Biol. 2000;3:254–261. [PubMed] [Google Scholar]
- 8.Ludewig U. Transfus Clin Biol. 2006;13:111–116. doi: 10.1016/j.tracli.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Ludewig U, Neuhäuser B, Dynowski M. FEBS Lett. 2007;581:2301–2308. doi: 10.1016/j.febslet.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Kustu S, Inwood W. Transfus Clin Biol. 2006;13:103–110. doi: 10.1016/j.tracli.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Soupene E, He L, Yan D, Kustu S. Proc Natl Acad Sci USA. 1998;95:7030–7034. doi: 10.1073/pnas.95.12.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soupene E, Ramirez RM, Kustu S. Mol Cell Biol. 2001;21:5733–5741. doi: 10.1128/MCB.21.17.5733-5741.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blakey D, Leech A, Thomas GH, Coutts G, Findlay K, Merrick M. Biochem J. 2002;364:527–535. doi: 10.1042/BJ20011761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guterman SK, Roberts G, Tyler B. J Bacteriol. 1982;150:1314–1321. doi: 10.1128/jb.150.3.1314-1321.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Mol Syst Biol. 2006;2 doi: 10.1038/msb4100050. 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harold FM. A Study of Bioenergetics. New York: Freeman; 1986. The Vital Force. [Google Scholar]
- 17.Walter A, Gutknecht J. J Membr Biol. 1986;90:207–217. doi: 10.1007/BF01870127. [DOI] [PubMed] [Google Scholar]
- 18.Colanduoni J, Nissan R, Villafranca JJ. J Biol Chem. 1987;262:3037–3043. [PubMed] [Google Scholar]
- 19.DeLano WL. The Pymol Molecular Graphics System. San Carlos, CA: DeLano Scientific; 2002. [Google Scholar]
- 20.Conroy MJ, Durand A, Lupo D, Li XD, Bullough PA, Winkler FK, Merrick M. Proc Natl Acad Sci USA. 2007;104:1213–1218. doi: 10.1073/pnas.0610348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javelle A, Lupo D, Zheng L, Li XD, Winkler FK, Merrick M. J Biol Chem. 2006;281:39492–39498. doi: 10.1074/jbc.M608325200. [DOI] [PubMed] [Google Scholar]
- 22.Eagleson M. Concise Encyclopedia of Chemistry. New York: de Gruyter; 1994. p. 66. [Google Scholar]
- 23.Cotton FA, Wilkinson G, Murillo CA, Bochmann M. Advanced Inorganic Chemistry. 6th Ed. New York: Wiley; 1999. p. 318. [Google Scholar]
- 24.Soupene E, King N, Feild E, Liu P, Niyogi KK, Huang CH, Kustu S. Proc Natl Acad Sci USA. 2002;99:7769–7773. doi: 10.1073/pnas.112225599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soupene E, Inwood W, Kustu S. Proc Natl Acad Sci USA. 2004;101:7787–7792. doi: 10.1073/pnas.0401809101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang CH, Peng J. Proc Natl Acad Sci USA. 2005;102:15512–15517. doi: 10.1073/pnas.0507886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endeward V, Cartron JP, Ripoche P, Gros G. Transfus Clin Biol. 2006;13:123–127. doi: 10.1016/j.tracli.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Mayer M, Dynowski M, Ludewig U. Biochem J. 2006;396:431–437. doi: 10.1042/BJ20060051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soupene E, van Heeswijk WC, Plumbridge J, Stewart V, Bertenthal D, Lee H, Prasad G, Paliy O, Charernnoppakul P, Kustu S. J Bacteriol. 2003;185:5611–5626. doi: 10.1128/JB.185.18.5611-5626.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neidhardt FC, Bloch PL, Smith DF. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lever JE. Anal Biochem. 1972;50:73–83. doi: 10.1016/0003-2697(72)90487-3. [DOI] [PubMed] [Google Scholar]
- 32.Rapp BJ, Landrum DC, Wall JD. Arch Microbiol. 1986;146:134–141. [Google Scholar]
- 33.Ikeda TP, Shauger AE, Kustu S. J Mol Biol. 1996;259:589–607. doi: 10.1006/jmbi.1996.0342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.