Abstract
Rhizobium meliloti 2011 DNA from pRmSL26, a plasmid which is known to carry genes involved in the early stages of nodulation, was used to construct Tn5 mutations by site-directed Tn5 mutagenesis. Tn5 mutations located within an 8.7 kilobase EcoRI fragment defined two adjacent loci encoding functions for nodulation (nod) and symbiotic N2 fixation (fix). We investigated the organization and regulation of the fix locus and the characteristics of alfalfa nodules induced by these Fix- mutants. By monitoring expression in Escherichia coli minicells, we determined that the fix locus encoded a 36-kilodalton polypeptide. The gene corresponding to this locus was designated fixF. Morphological and ultrastructural studies of the ineffective nodules formed by R. meliloti fixF mutants showed infected host cells similar to those of the wild type. The ineffective nodules were able to accumulate leghemoglobin, but at lower levels than those found in the wild-type nodules. Expression of the nifHDK operon was unaffected by Tn5 insertions in the fixF gene. Expression of the fixF gene was monitored in E. coli by using translational lacZ fusions. It was shown that transcription of the fixF gene in E. coli could be activated by Klebsiella pneumoniae nifA and the R. meliloti nifA-like regulatory gene products. Expression of the fixF gene was also studied in free-living and symbiotic R. meliloti cells. It was found that the fixF gene was transcribed in the symbiotic state.
Full text
PDF






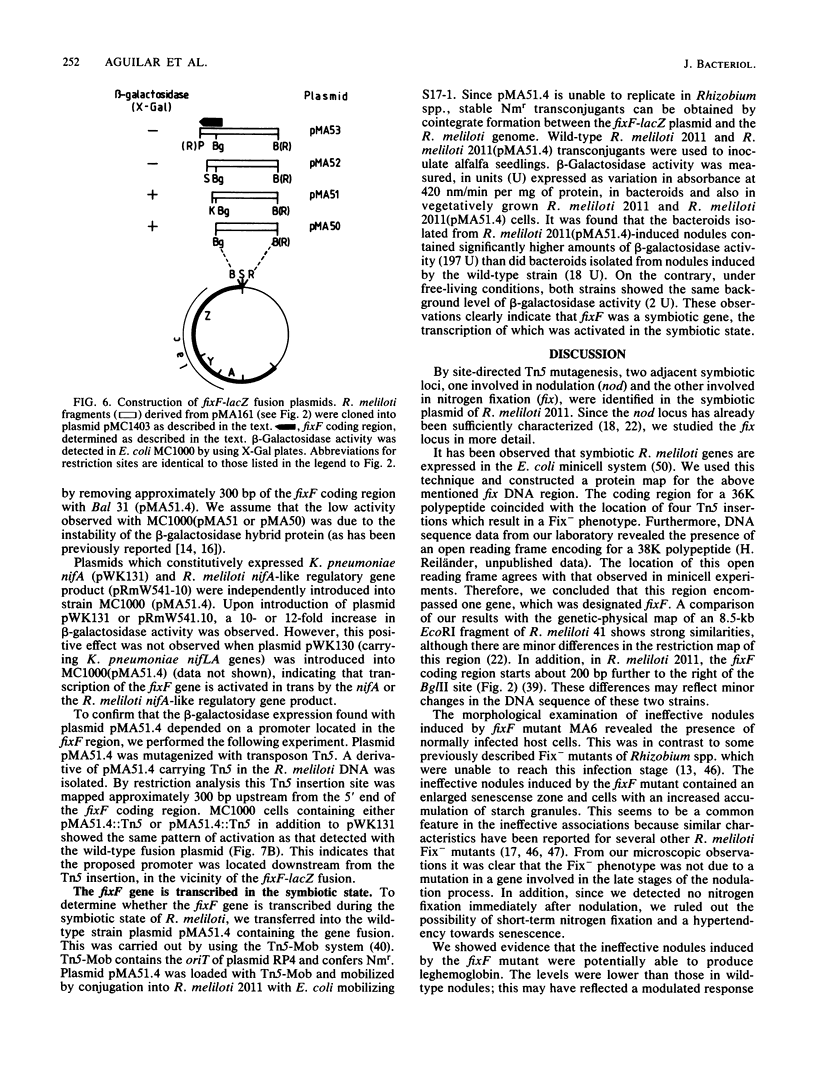


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar Y. J., Nadler K. D. Stimulation of tetrapyrrole formation in Rhizobium japonicum by restricted aeration. J Bacteriol. 1978 Sep;135(3):782–789. doi: 10.1128/jb.135.3.782-789.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Bisseling T., van den Bos R. C., van Kammen A. The effect of ammonium nitrate on the synthesis of nitrogenase and the concentration of leghemoglobin in pea root nodules induced by Rhizobium leguminosarum. Biochim Biophys Acta. 1978 Feb 13;539(1):1–11. doi: 10.1016/0304-4165(78)90115-0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Bánfalvi Z., Sakanyan V., Koncz C., Kiss A., Dusha I., Kondorosi A. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of R. meliloti. Mol Gen Genet. 1981;184(2):318–325. doi: 10.1007/BF00272925. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The site of heme synthesis in soybean root nodules. Biochim Biophys Acta. 1969 Dec 30;192(3):486–493. doi: 10.1016/0304-4165(69)90398-5. [DOI] [PubMed] [Google Scholar]
- Dixon R. A. The genetic complexity of nitrogen fixation. The ninth Fleming lecture. J Gen Microbiol. 1984 Nov;130(11):2745–2755. doi: 10.1099/00221287-130-11-2745. [DOI] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Ma Q. S., Knight C. D., Hombrecher G., Johnston A. W. Cloning of the symbiotic region of Rhizobium leguminosarum: the nodulation genes are between the nitrogenase genes and a nifA-like gene. EMBO J. 1983;2(6):947–952. doi: 10.1002/j.1460-2075.1983.tb01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Silhavy T. J. Mutations affecting localization of an Escherichia coli outer membrane protein, the bacteriophage lambda receptor. J Mol Biol. 1980 Jul 25;141(1):63–90. doi: 10.1016/s0022-2836(80)80029-5. [DOI] [PubMed] [Google Scholar]
- Goldbach R. W., Borst P., Bollen-de Boer J. E., van Bruggen E. F. The organization of ribosomal RNA genes in the mitochondrial DNA of Tetrahymena pyriformis strain ST. Biochim Biophys Acta. 1978 Nov 21;521(1):169–186. doi: 10.1016/0005-2787(78)90260-5. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Müller-Hill B. Synthetic multifunctional proteins: isolation of covalently linked tryptophan synthetase alpha-subunit-lac-repressor-beta-galactosidase chimeras. Mol Gen Genet. 1977 Oct 24;155(3):301–307. doi: 10.1007/BF00272809. [DOI] [PubMed] [Google Scholar]
- Hirsch A. M., Bang M., Ausubel F. M. Ultrastructural analysis of ineffective alfalfa nodules formed by nif::Tn5 mutants of Rhizobium meliloti. J Bacteriol. 1983 Jul;155(1):367–380. doi: 10.1128/jb.155.1.367-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. M., Drake D., Jacobs T. W., Long S. R. Nodules are induced on alfalfa roots by Agrobacterium tumefaciens and Rhizobium trifolii containing small segments of the Rhizobium meliloti nodulation region. J Bacteriol. 1985 Jan;161(1):223–230. doi: 10.1128/jb.161.1.223-230.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN D. C., GRINYER I., COULTER W. H. ELECTRON MICROSCOPY OF INFECTION THREADS AND BACTERIA IN YOUNG ROOT NODULES OF MEDICAGO SATIVA. J Bacteriol. 1963 Jul;86:125–137. doi: 10.1128/jb.86.1.125-137.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A. J., Hontelez J. G., Van den Bos R. C., Van Kammen A. Expression of large plasmids in the endosymbiotic form of Rhizobium leguminosarum. Nucleic Acids Res. 1980 Oct 10;8(19):4337–4347. doi: 10.1093/nar/8.19.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Inouye M. Construction of versatile expression cloning vehicles using the lipoprotein gene of Escherichia coli. EMBO J. 1982;1(6):771–775. doi: 10.1002/j.1460-2075.1982.tb01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Stacey G., Tandon S. R., Silver L. E., Brill W. J. Rhizobium japonicum mutants defective in symbiotic nitrogen fixation. J Bacteriol. 1982 Oct;152(1):485–494. doi: 10.1128/jb.152.1.485-494.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Paau A. S., Bloch C. B., Brill W. J. Developmental fate of Rhizobium meliloti bacteroids in alfalfa nodules. J Bacteriol. 1980 Sep;143(3):1480–1490. doi: 10.1128/jb.143.3.1480-1490.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C., Boistard P., Dénarié J., Casse-Delbart F. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184(2):326–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J., John M., Kondorosi E., Kondorosi A., Wieneke U., Schröder G., Schröder J., Schell J. Mapping of the protein-coding regions of Rhizobium meliloti common nodulation genes. EMBO J. 1984 Aug;3(8):1705–1711. doi: 10.1002/j.1460-2075.1984.tb02035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. High frequency mobilization of gram-negative bacterial replicons by the in vitro constructed Tn5-Mob transposon. Mol Gen Genet. 1984;196(3):413–420. doi: 10.1007/BF00436188. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Sundaresan V., Jones J. D., Ow D. W., Ausubel F. M. Klebsiella pneumoniae nifA product activates the Rhizobium meliloti nitrogenase promoter. Nature. 1983 Feb 24;301(5902):728–732. doi: 10.1038/301728a0. [DOI] [PubMed] [Google Scholar]
- Szeto W. W., Zimmerman J. L., Sundaresan V., Ausubel F. M. A Rhizobium meliloti symbiotic regulatory gene. Cell. 1984 Apr;36(4):1035–1043. doi: 10.1016/0092-8674(84)90053-9. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. L., Szeto W. W., Ausubel F. M. Molecular characterization of Tn5-induced symbiotic (Fix-) mutants of Rhizobium meliloti. J Bacteriol. 1983 Dec;156(3):1025–1034. doi: 10.1128/jb.156.3.1025-1034.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]





