Abstract
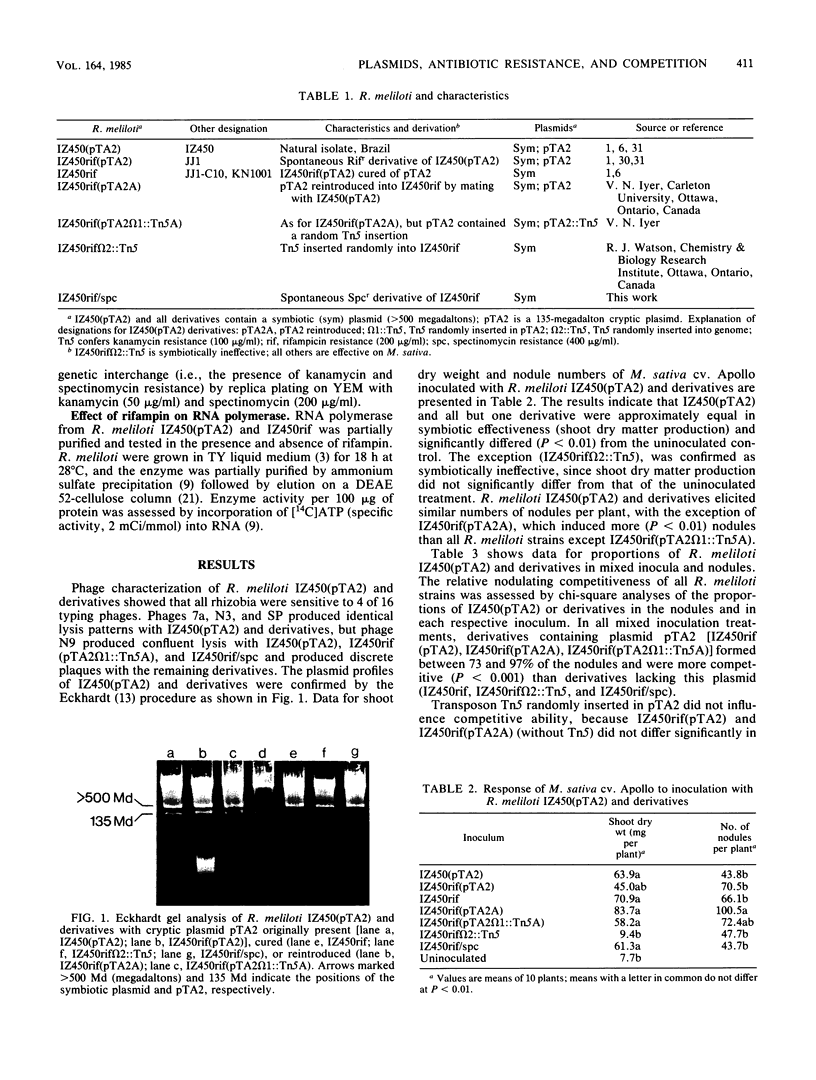
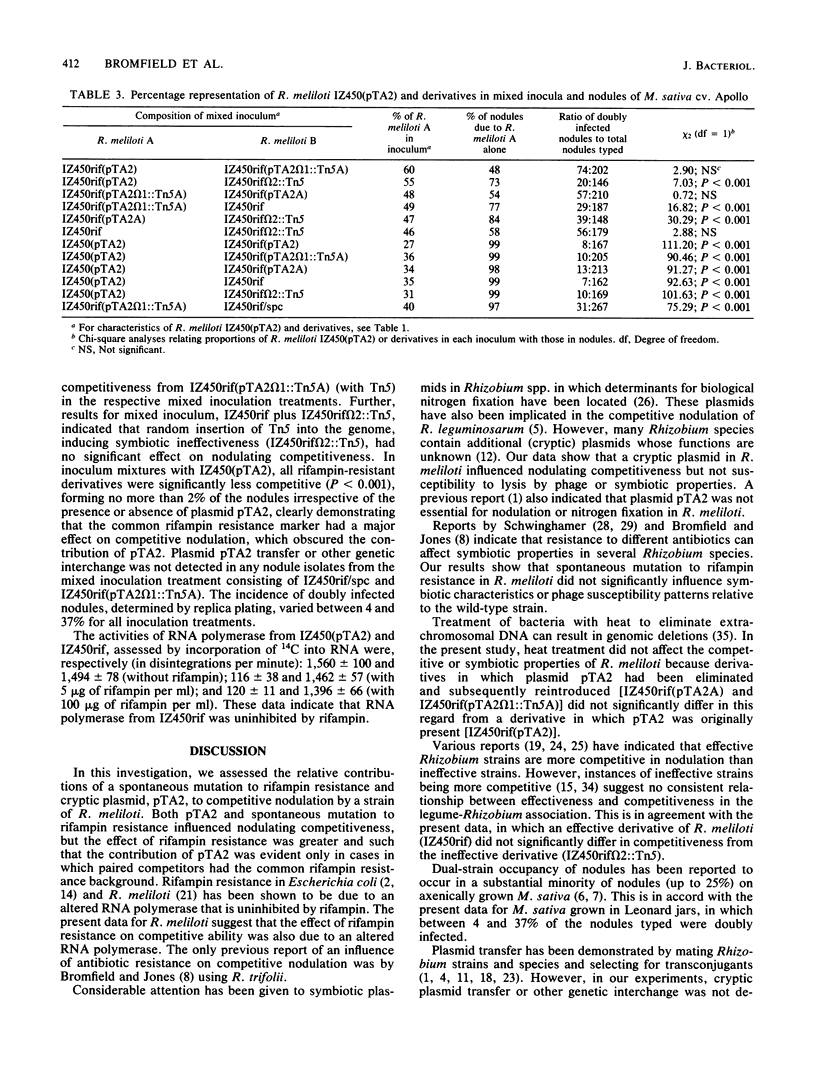
An assessment was made of the relative contributions of a spontaneous mutation to rifampin resistance and a cryptic plasmid, pTA2, to competitive nodulation of Medicago sativa by a strain of Rhizobium meliloti. This was facilitated by use of rifampin-resistant derivatives of this strain in which pTA2 was originally present, cured, or reintroduced. Both curing of pTA2 and spontaneous mutation to rifampin resistance significantly influenced nodulating competitiveness, but the effect of rifampin resistance was greater and such that the contribution of pTA2 was evident only in cases in which paired competitors had the common rifampin resistance background. The data suggest that rifampin-resistant derivatives contain an altered RNA polymerase insensitive to the action of rifampin. All R. meliloti derivatives had symbiotic characteristics and phage susceptibility patterns similar to those of the wild type. Plasmid pTA2 transfer or other genetic interchange was not detected in nodules of M. sativa inoculated with paired competitors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin S., Scaife J. A new method for selecting RNA polymerase mutants. J Mol Biol. 1970 Apr 14;49(1):263–267. doi: 10.1016/0022-2836(70)90394-3. [DOI] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Bromfield E. S. Variation in Preference for Rhizobium meliloti Within and Between Medicago sativa Cultivars Grown in Soil. Appl Environ Microbiol. 1984 Dec;48(6):1231–1236. doi: 10.1128/aem.48.6.1231-1236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Ezekiel D. H., Hutchins J. E. Mutations affecting RNA polymerase associated with rifampicin resistance in Escherichia coli. Nature. 1968 Oct 19;220(5164):276–277. doi: 10.1038/220276a0. [DOI] [PubMed] [Google Scholar]
- Nielsen B. L., Brown L. R. Purification and subunit characterization of Rhizobium meliloti RNA polymerase. J Bacteriol. 1985 May;162(2):645–650. doi: 10.1128/jb.162.2.645-650.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilacinski W. P., Schmidt E. L. Plasmid transfer within and between serologically distinct strains of Rhizobium japonicum, using antibiotic resistance mutants and auxotrophs. J Bacteriol. 1981 Feb;145(2):1025–1030. doi: 10.1128/jb.145.2.1025-1030.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWINGHAMER E. A. ASSOCIATION BETWEEN ANTIBIOTIC RESISTANCE AND INEFFECTIVENESS IN MUTANT STRAINS OF RHIZOBIUM SPP. Can J Microbiol. 1964 Apr;10:221–233. doi: 10.1139/m64-029. [DOI] [PubMed] [Google Scholar]
- Schwinghamer E. A. Effectiveness of Rhizobium as modified by mutation for resistance to antibiotics. Antonie Van Leeuwenhoek. 1967;33(2):121–136. doi: 10.1007/BF02045542. [DOI] [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. Suicide plasmid vehicles for insertion mutagenesis in Rhizobium meliloti and related bacteria. J Bacteriol. 1983 Dec;156(3):1292–1300. doi: 10.1128/jb.156.3.1292-1300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. Transposon Tn5 specifies streptomycin resistance in Rhizobium spp. J Bacteriol. 1984 May;158(2):580–589. doi: 10.1128/jb.158.2.580-589.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINCENT J. M., WATERS L. M. The influence of the host on competition amongst clover root-nodule bacteria. J Gen Microbiol. 1953 Dec;9(3):357–370. doi: 10.1099/00221287-9-3-357. [DOI] [PubMed] [Google Scholar]
- Zurkowski W. Molecular mechanism for loss of nodulation properties of Rhizobium trifolii. J Bacteriol. 1982 Jun;150(3):999–1007. doi: 10.1128/jb.150.3.999-1007.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



