Abstract
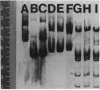
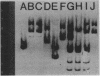
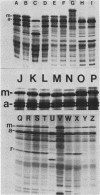
A 4.84-kilobase-pair plasmid was isolated from Proteus vulgaris (ATCC 13315) and cloned into the plasmid vector pBR322. Plasmid pBR322 contains substrate sites for the restriction endonucleases PvuI and PvuII. The recombinant plasmids were resistant to in vitro cleavage by PvuII but not PvuI endonuclease and were found to cause production of PvuII endonuclease or methylase activity or both in Escherichia coli HB101. The approximate endonuclease and methylase gene boundaries were determined through subcloning, Bal 31 resection, insertional inactivation, DNA-dependent translation, and partial DNA sequencing. The two genes are adjacent and appear to be divergently transcribed. Most E. coli strains tested were poorly transformed by the recombinant plasmids, and this was shown by subcloning and insertional inactivation to be due to the PvuII methylase gene. At a low frequency, stable methylase-producing transformants of a methylase-sensitive strain were obtained, and efficiently transformed cell mutants were isolated from them.
Full text
PDF

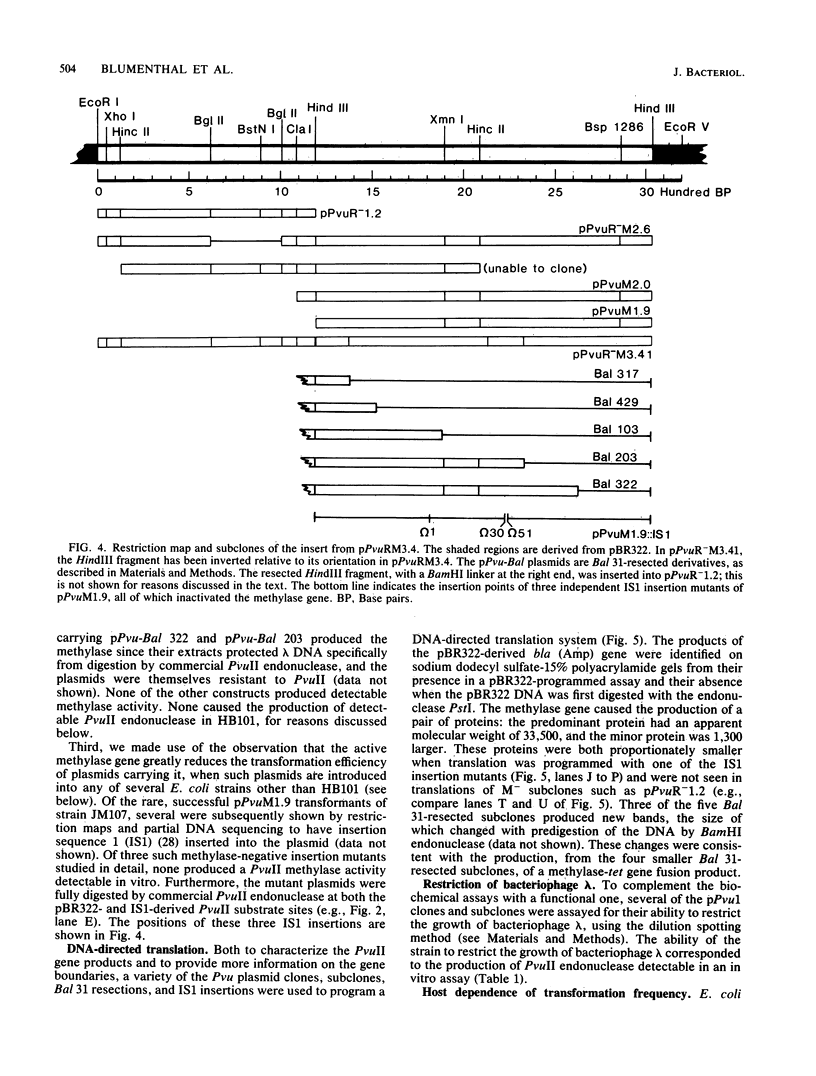
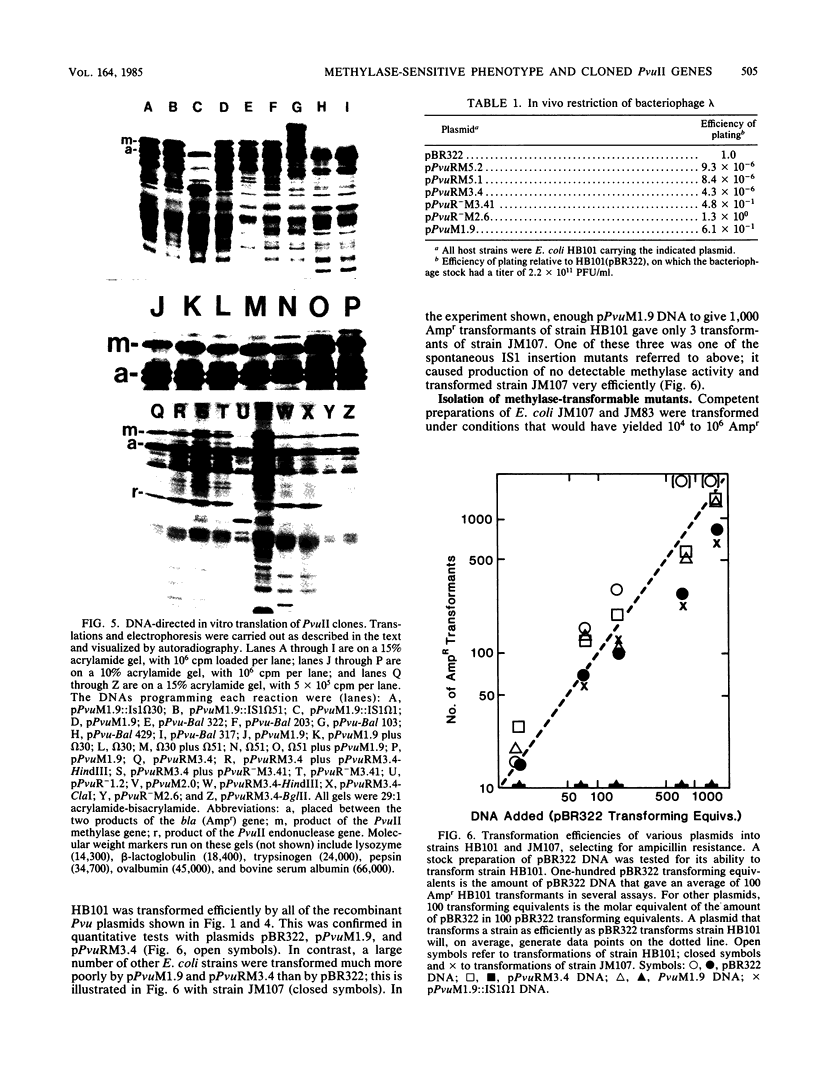




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberg S., Dennison S. Variation in expression of sex factor genes in the Proteus-Providencia group relative to Escherichia coli. J Bacteriol. 1975 Jul;123(1):278–286. doi: 10.1128/jb.123.1.278-286.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bougueleret L., Schwarzstein M., Tsugita A., Zabeau M. Characterization of the genes coding for the Eco RV restriction and modification system of Escherichia coli. Nucleic Acids Res. 1984 Apr 25;12(8):3659–3676. doi: 10.1093/nar/12.8.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. E., Roberts R. J. Modification profiles of bacterial genomes. Nucleic Acids Res. 1982 Feb 11;10(3):913–934. doi: 10.1093/nar/10.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby C., Jr, Hu A. S. The regulation of the synthesis of beta-galactosidase in Proteus mirabilis F-lac. Biochim Biophys Acta. 1968 Mar 18;157(1):149–158. doi: 10.1016/0005-2787(68)90273-6. [DOI] [PubMed] [Google Scholar]
- Dobritsa A. P., Dobritsa S. V. DNA protection with the DNA methylase M . BbvI from Bacillus brevis var. GB against cleavage by the restriction endonucleases PstI and PvuII. Gene. 1980 Jul;10(2):105–112. doi: 10.1016/0378-1119(80)90128-6. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Gama-Sosa M. A., Carreira L. H., Ljungdahl L. G., Kuo K. C., Gehrke C. W. DNA methylation in thermophilic bacteria: N4-methylcytosine, 5-methylcytosine, and N6-methyladenine. Nucleic Acids Res. 1985 Feb 25;13(4):1399–1412. doi: 10.1093/nar/13.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Brooks J. E. Cloned restriction/modification system from Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1983 Jan;80(2):402–406. doi: 10.1073/pnas.80.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Greenough L., Schildkraut I., Roberts R. J. Two new restriction endonucleases from Proteus vulgaris. Nucleic Acids Res. 1981 Sep 25;9(18):4525–4536. doi: 10.1093/nar/9.18.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günthert U., Freund M., Trautner T. A. Restriction and modification in Bacillus subtilis: two DNA methyltransferases with BsuRI specificity. I. Purification and physical properties. J Biol Chem. 1981 Sep 10;256(17):9340–9345. [PubMed] [Google Scholar]
- Günthert U., Jentsch S., Freund M. Restriction and modification in Bacillus subtilis: two DNA methyltransferases with BsuRI specificity. II. Catalytic properties, substrate specificity, and mode of action. J Biol Chem. 1981 Sep 10;256(17):9346–9351. [PubMed] [Google Scholar]
- Herman G. E., Modrich P. Escherichia coli dam methylase. Physical and catalytic properties of the homogeneous enzyme. J Biol Chem. 1982 Mar 10;257(5):2605–2612. [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Bickle T. A. Bacteriophage survival: multiple mechanisms for avoiding the deoxyribonucleic acid restriction systems of their hosts. Microbiol Rev. 1983 Sep;47(3):345–360. doi: 10.1128/mr.47.3.345-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. A. Purification and properties of the complementary endonucleases DpnI and DpnII. Methods Enzymol. 1980;65(1):138–146. doi: 10.1016/s0076-6879(80)65019-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Manson M. D., Yanofsky C. Tryptophan operon regulation in interspecific hybrids of enteric bacteria. J Bacteriol. 1976 May;126(2):679–689. doi: 10.1128/jb.126.2.679-689.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ohtsubo H., Ohtsubo E. Nucleotide sequence of an insertion element, IS1. Proc Natl Acad Sci U S A. 1978 Feb;75(2):615–619. doi: 10.1073/pnas.75.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. A., Modrich P. EcoRI methylase. Physical and catalytic properties of the homogeneous enzyme. J Biol Chem. 1977 Oct 25;252(20):7265–7272. [PubMed] [Google Scholar]
- Smith J. T. R-factor gene expression gram-negative bacteria. J Gen Microbiol. 1969 Jan;55(1):109–120. doi: 10.1099/00221287-55-1-109. [DOI] [PubMed] [Google Scholar]
- Smith L. A., Chirikjian J. G. Purification and characterization of the sequence-specific endonuclease Bam HI. J Biol Chem. 1979 Feb 25;254(4):1003–1006. [PubMed] [Google Scholar]
- Walder R. Y., Hartley J. L., Donelson J. E., Walder J. A. Cloning and expression of the Pst I restriction-modification system in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1503–1507. doi: 10.1073/pnas.78.3.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder R. Y., Walder J. A., Donelson J. E. The organization and complete nucleotide sequence of the PstI restriction-modification system. J Biol Chem. 1984 Jun 25;259(12):8015–8026. [PubMed] [Google Scholar]
- Wang R. Y., Shenoy S., Ehrlich M. DNA methylation inhibits the transfecting activity of replicative- form phi X174 DNA. J Virol. 1984 Mar;49(3):674–679. doi: 10.1128/jvi.49.3.674-679.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yoo O. J., Agarwal K. L. Isolation and characterization of two proteins possessing Hpa II methylase activity. J Biol Chem. 1980 Jul 10;255(13):6445–6449. [PubMed] [Google Scholar]
- Yuan R. Structure and mechanism of multifunctional restriction endonucleases. Annu Rev Biochem. 1981;50:285–319. doi: 10.1146/annurev.bi.50.070181.001441. [DOI] [PubMed] [Google Scholar]