Abstract
Translational coupling was demonstrated in a gene fusion in which the promoter and the N-terminal region of the Bacillus subtilis subtilisin (aprA) gene were fused to a promoterless Tn9-derived chloramphenicol acetyltransferase (CAT; EC 2.3.1.28) gene. Expression of this gene fusion results in the production of a native-sized CAT product, whereas the Tn9-derived CAT gene is usually not translated from its own ribosome binding site in B. subtilis (D. S. Goldfarb, R. L. Rodriguez, and R. H. Doi, Proc. Natl. Acad. Sci. USA 79:5886-5890, 1982). A 178-base-pair deletion, which removed part of the signal peptide and the propeptide of the aprA gene and created a translational stop codon 230 base pairs upstream of the CAT gene ribosome binding site, reduced expression of the CAT gene. A BamHI 10-mer linker insertion into this deletion site, which restored the reading frame and simultaneously removed the translation stop codon, restored CAT gene expression. The data indicate that expression of the CAT gene was dependent on translation of the truncated aprA gene into the ribosome binding site of the CAT gene.
Full text
PDF
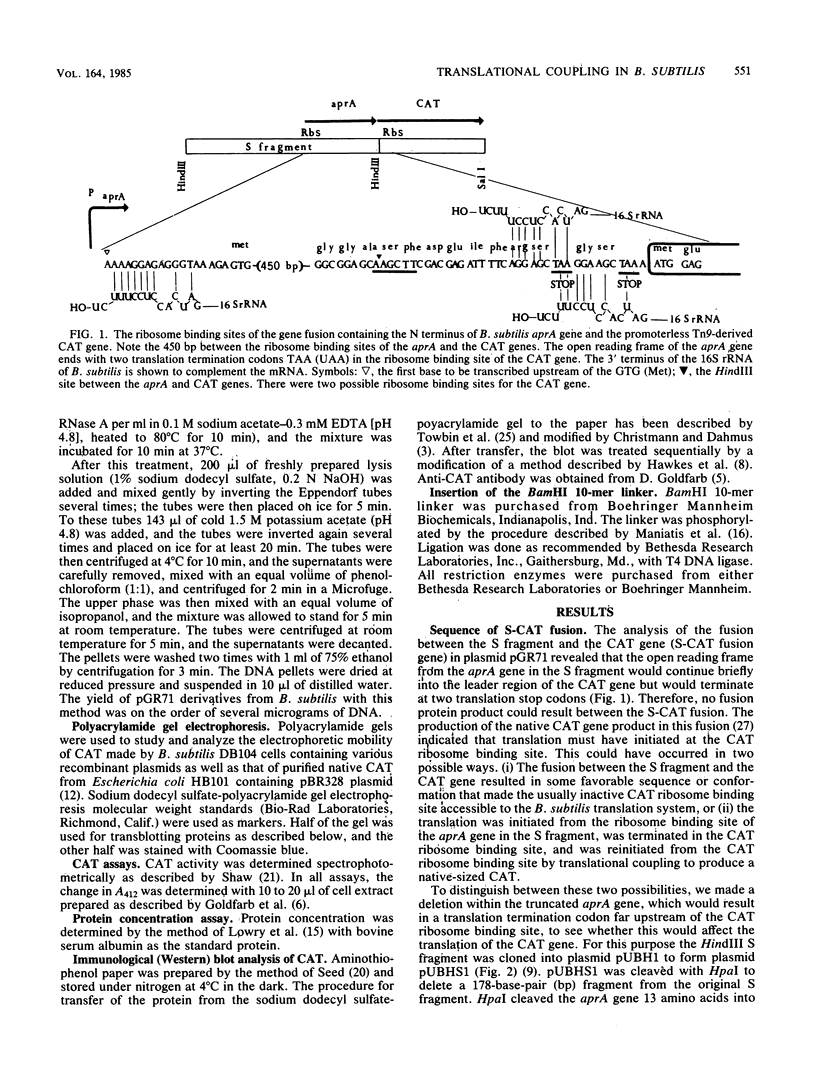
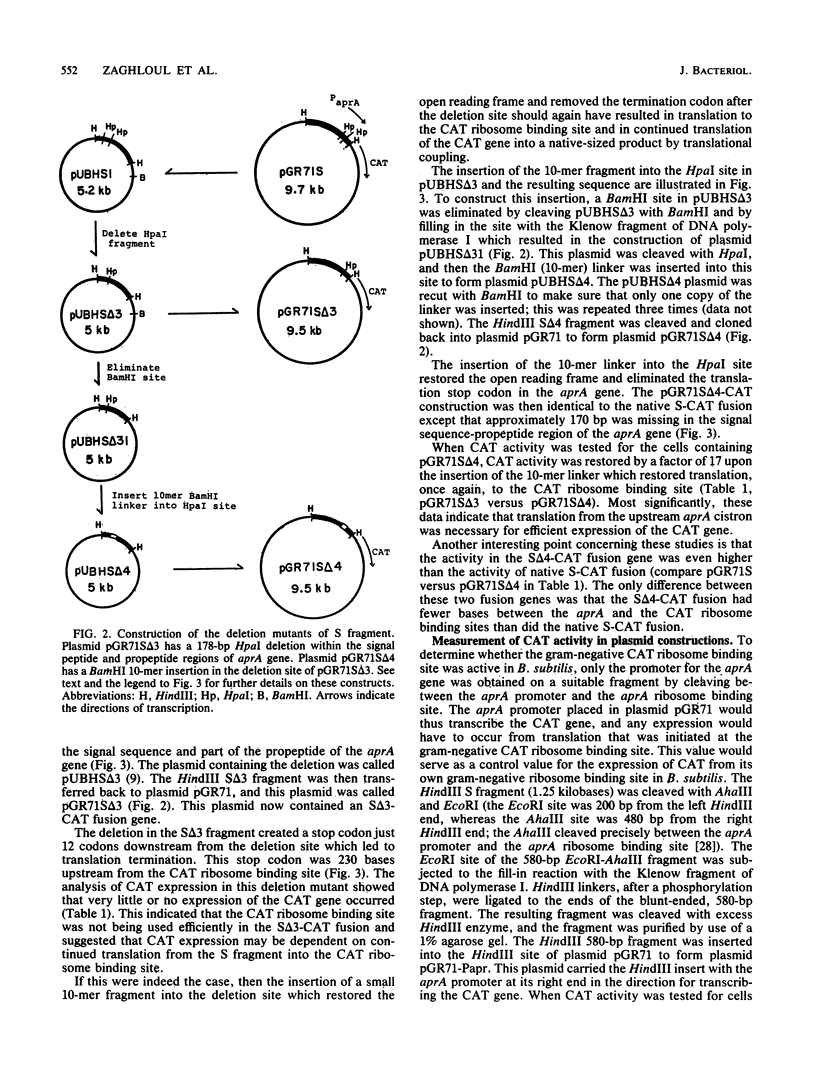
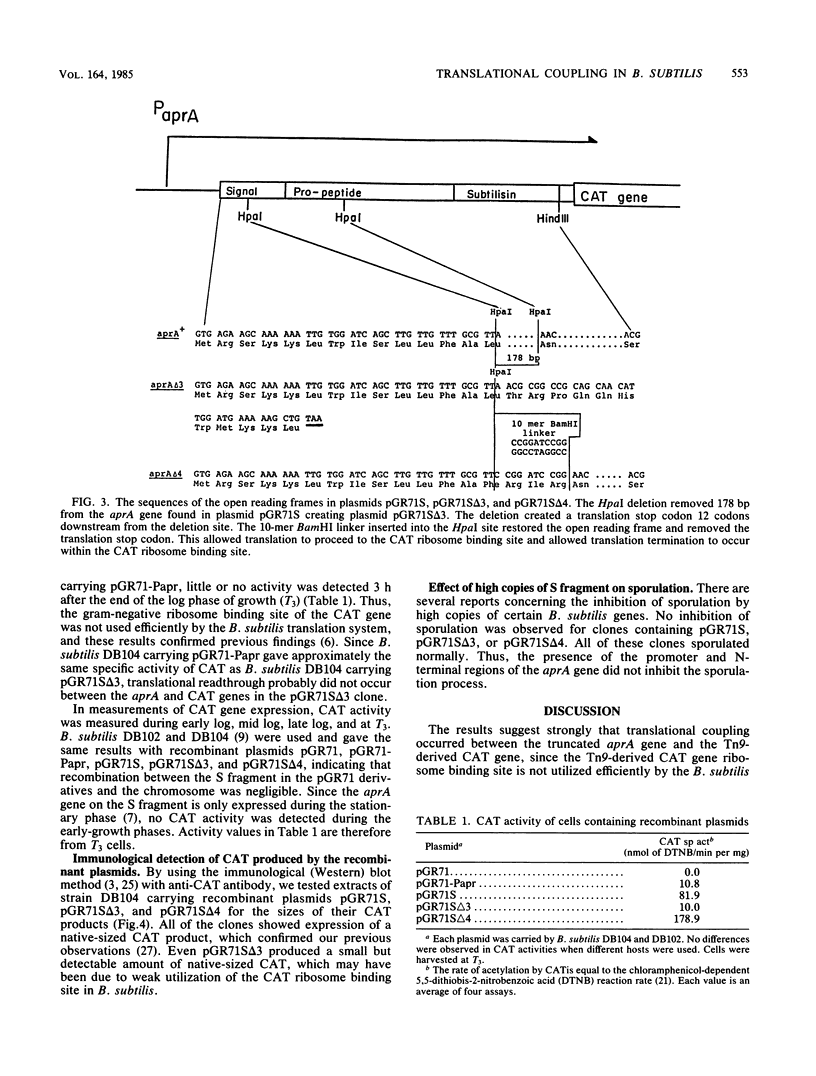


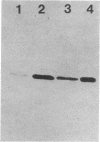
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksoy S., Squires C. L., Squires C. Translational coupling of the trpB and trpA genes in the Escherichia coli tryptophan operon. J Bacteriol. 1984 Feb;157(2):363–367. doi: 10.1128/jb.157.2.363-367.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman G., Nomura M. Localization of the target site for translational regulation of the L11 operon and direct evidence for translational coupling in Escherichia coli. Cell. 1983 Oct;34(3):979–988. doi: 10.1016/0092-8674(83)90555-x. [DOI] [PubMed] [Google Scholar]
- Christmann J. L., Dahmus M. E. Monoclonal antibody specific for calf thymus RNA polymerases IIO and IIA. J Biol Chem. 1981 Nov 25;256(22):11798–11803. [PubMed] [Google Scholar]
- Das A., Yanofsky C. A ribosome binding site sequence is necessary for efficient expression of the distal gene of a translationally-coupled gene pair. Nucleic Acids Res. 1984 Jun 11;12(11):4757–4768. doi: 10.1093/nar/12.11.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb D. S., Doi R. H., Rodriguez R. L. Expression of Tn9-derived chloramphenicol resistance in Bacillus subtilis. Nature. 1981 Sep 24;293(5830):309–311. doi: 10.1038/293309a0. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S., Rodriguez R. L., Doi R. H. Translational block to expression of the Escherichia coli Tn9-derived chloramphenicol-resistance gene in Bacillus subtilis. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5886–5890. doi: 10.1073/pnas.79.19.5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb D. S., Wong S. L., Kudo T., Doi R. H. A temporally regulated promoter from Bacillus subtilis is transcribed only by an RNA polymerase with a 37,000 dalton sigma factor. Mol Gen Genet. 1983;191(2):319–325. doi: 10.1007/BF00334833. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Moran C. P., Jr, Losick R. Two RNA polymerase sigma factors from Bacillus subtilis discriminate between overlapping promoters for a developmentally regulated gene. Nature. 1983 Apr 28;302(5911):800–804. doi: 10.1038/302800a0. [DOI] [PubMed] [Google Scholar]
- Kawamura F., Doi R. H. Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J Bacteriol. 1984 Oct;160(1):442–444. doi: 10.1128/jb.160.1.442-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura F., Saito H., Ikeda Y. Bacteriophage phi 1 as a gene-cloning vector in Bacillus subtilis. Mol Gen Genet. 1980;180(2):259–266. doi: 10.1007/BF00425837. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Lin C. K., Goldfarb D. S., Doi R. H., Rodriguez R. L. Mutations that affect the translation efficiency of Tn9-derived cat gene in Bacillus subtilis. Proc Natl Acad Sci U S A. 1985 Jan;82(1):173–177. doi: 10.1073/pnas.82.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980 Aug;95(4):785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs D., Artz S. The hisD-hisC gene border of the Salmonella typhimurium histidine operon. Mol Gen Genet. 1984;196(3):526–529. doi: 10.1007/BF00436203. [DOI] [PubMed] [Google Scholar]
- Schümperli D., McKenney K., Sobieski D. A., Rosenberg M. Translational coupling at an intercistronic boundary of the Escherichia coli galactose operon. Cell. 1982 Oct;30(3):865–871. doi: 10.1016/0092-8674(82)90291-4. [DOI] [PubMed] [Google Scholar]
- Seed B. Diazotizable arylamine cellulose papers for the coupling and hybridization of nucleic acids. Nucleic Acids Res. 1982 Mar 11;10(5):1799–1810. doi: 10.1093/nar/10.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel R., Reiss B., Schaller H. Translationally coupled initiation of protein synthesis in Bacillus subtilis. Nucleic Acids Res. 1985 Feb 11;13(3):893–909. doi: 10.1093/nar/13.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. Z., Doi R. H. Overlapping promoters transcribed by bacillus subtilis sigma 55 and sigma 37 RNA polymerase holoenzymes during growth and stationary phases. J Biol Chem. 1984 Jul 10;259(13):8619–8625. [PubMed] [Google Scholar]
- Wong S. L., Price C. W., Goldfarb D. S., Doi R. H. The subtilisin E gene of Bacillus subtilis is transcribed from a sigma 37 promoter in vivo. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1184–1188. doi: 10.1073/pnas.81.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]