Abstract
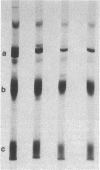
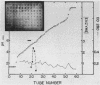
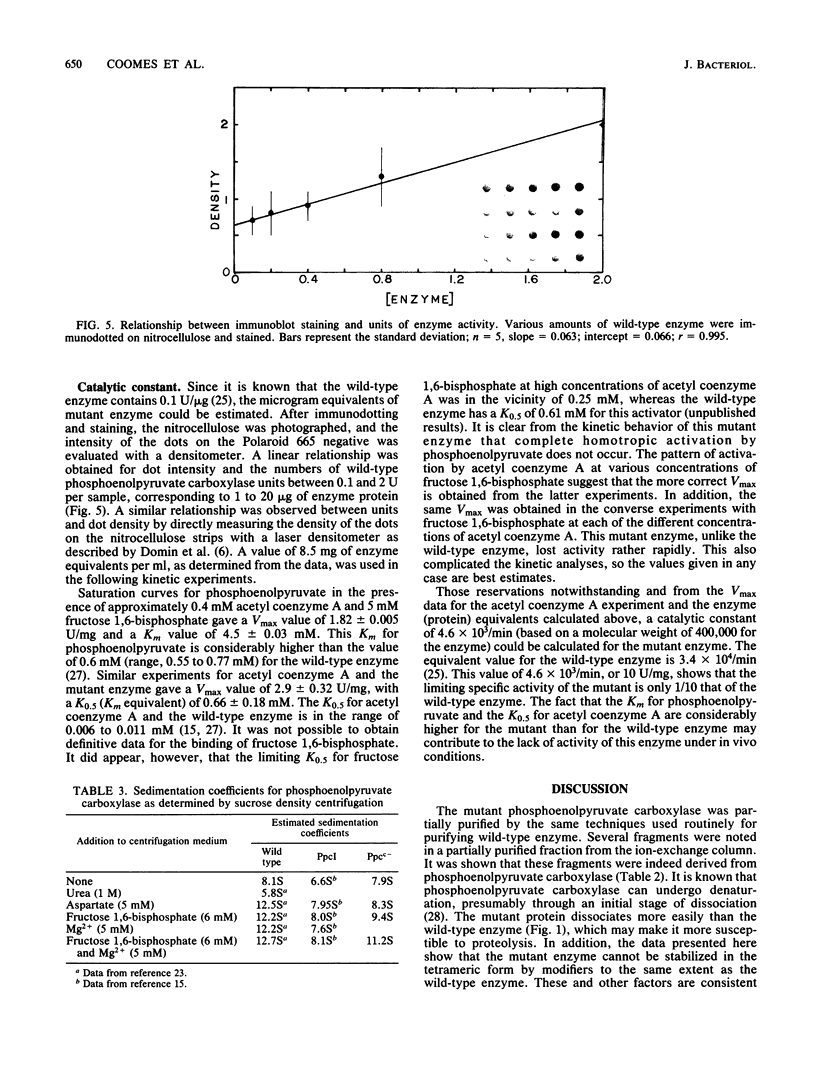
A mutant Escherichia coli (Ppcc-) which was unable to grow on glucose as a sole carbon source was isolated. This mutant had very low levels of phosphoenolpyruvate carboxylase activity (approximately 5% of the wild type). Goat immunoglobulin G prepared against wild-type phosphoenolypyruvate carboxylase cross-reacted with the Ppcc- enzyme. The amount of enzyme protein in the mutant cells was similar to that found in wild-type cells, but it had greatly diminished specific activity. The catalytically less active mutant enzyme retained the ability to interact with fructose 1,6-bisphosphate, but did not exhibit stabilization of the tetrameric form by aspartate. The pI of the mutant protein was lower (4.9) than that of the wild-type protein (5.1). After electrophoresis and immunoblotting of the partially purified protein, several immunostaining bands were seen in addition to the main enzyme band. A novel method for showing that these bands represented proteolytic fragments of phosphoenolpyruvate carboxylase was developed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin L. M., Fanning G. R. Studies of parameters affecting the allosteric nature of phosphoenolpyruvate carboxylase of Escherichia coli. J Biol Chem. 1968 Jun 25;243(12):3517–3525. [PubMed] [Google Scholar]
- Cánovas J. L., Kornberg H. L. Properties and regulation of phosphopyruvate carboxylase activity in Escherichia coli. Proc R Soc Lond B Biol Sci. 1966 Aug 16;165(999):189–205. doi: 10.1098/rspb.1966.0064. [DOI] [PubMed] [Google Scholar]
- Domin B. A., Serabjit-Singh C. J., Philpot R. M. Quantitation of rabbit cytochrome P-450, form 2, in microsomal preparations bound directly to nitrocellulose paper using a modified peroxidase-immunostaining procedure. Anal Biochem. 1984 Feb;136(2):390–396. doi: 10.1016/0003-2697(84)90234-3. [DOI] [PubMed] [Google Scholar]
- Fujita N., Miwa T., Ishijima S., Izui K., Katsuki H. The primary structure of phosphoenolpyruvate carboxylase of Escherichia coli. Nucleotide sequence of the ppc gene and deduced amino acid sequence. J Biochem. 1984 Apr;95(4):909–916. doi: 10.1093/oxfordjournals.jbchem.a134718. [DOI] [PubMed] [Google Scholar]
- Izui K., Nishikido T., Ishihara K., Katsuki H. Studies on the allosteric effectors and some properties of phosphoenolpyruvate carboxylase from Escherichia coli. J Biochem. 1970 Aug;68(2):215–226. doi: 10.1093/oxfordjournals.jbchem.a129349. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McAlister L. E., Evans E. L., Smith T. E. Properties of a mutant Escherichia coli phosphoenolpyruvate carboxylase deficient in coregulation by intermediary metabolites. J Bacteriol. 1981 Apr;146(1):200–208. doi: 10.1128/jb.146.1.200-208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Morikawa M., Izui K., Katsuki H. Phosphoenolpyruvate carboxylase of E. coli: discrimination of regulatory sites for four kinds of allosteric effectors by the method of genetic desensitization. Biochem Biophys Res Commun. 1971 Nov 5;45(3):689–694. doi: 10.1016/0006-291x(71)90471-2. [DOI] [PubMed] [Google Scholar]
- Morikawa M., Izui K., Katsuki H. Studies on the allosteric properties of mutationally altered phosphoenolpyruvate carboxylases of Escherichia coli. Discrimination of allosteric sites. J Biochem. 1977 May;81(5):1473–1485. [PubMed] [Google Scholar]
- SADLER J. R., NOVICK A. THE PROPERTIES OF REPRESSOR AND THE KINETICS OF ITS ACTION. J Mol Biol. 1965 Jun;12:305–327. doi: 10.1016/s0022-2836(65)80255-8. [DOI] [PubMed] [Google Scholar]
- Sanwal B. D., Maeba P. Phosphoenolpyruvate carboxylase: activation by nucleotides as a possible compensatory feedback effect. J Biol Chem. 1966 Oct 10;241(19):4557–4562. [PubMed] [Google Scholar]
- Sanwal B. D., Maeba P. Regulation of the activity of phosphoenolypyruvate carboxylase by fructose diphosphate. Biochem Biophys Res Commun. 1966 Jan 24;22(2):194–199. doi: 10.1016/0006-291x(66)90431-1. [DOI] [PubMed] [Google Scholar]
- Smith T. E., Balasubramanian K. A., Beezley A. Escherichia coli phosphoenolpyruvate carboxylase. Studies on the mechanism of synergistic activation by nucleotides. J Biol Chem. 1980 Feb 25;255(4):1635–1642. [PubMed] [Google Scholar]
- Smith T. E. Escherichia coli phosphoenolpyruvate carboxylase. Physical and chemical properties. J Biol Chem. 1971 Jul 10;246(13):4234–4241. [PubMed] [Google Scholar]
- Smith T. E. Escherichia coli phosphoenolpyruvate carboxylase: characterization and sedimentation behavior. Arch Biochem Biophys. 1968 Dec;128(3):611–622. doi: 10.1016/0003-9861(68)90071-4. [DOI] [PubMed] [Google Scholar]
- Smith T. E. Escherichia coli phosphoenolpyruvate carboxylase: competitive regulation by acetyl-coenzyme A and aspartate. Arch Biochem Biophys. 1970 Apr;137(2):512–522. doi: 10.1016/0003-9861(70)90469-8. [DOI] [PubMed] [Google Scholar]
- Smith T. E. Escherichia coli phosphoenolpyruvate carboxylase: studies on the mechanism of multiple allosteric interactions. Arch Biochem Biophys. 1977 Oct;183(2):538–552. doi: 10.1016/0003-9861(77)90389-7. [DOI] [PubMed] [Google Scholar]
- THEODORE T. S., ENGLESBERG E. MUTANT OF SALMONELLA TYPHIMURIUM DEFICIENT IN THE CARBON DIOXIDE-FIXING ENZYME PHOSPHOENOLPYRUVIC CARBOXYLASE. J Bacteriol. 1964 Oct;88:946–955. doi: 10.1128/jb.88.4.946-955.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]