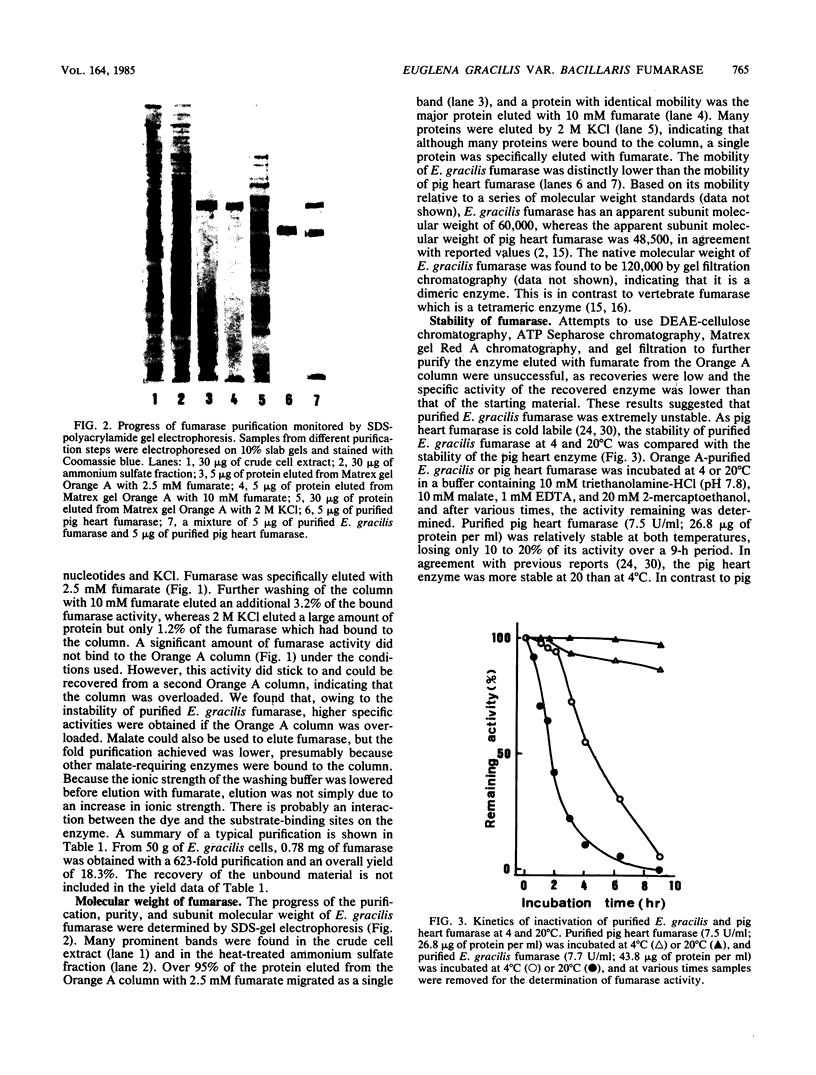
Abstract
A rapid three-step procedure utilizing heat treatment, ammonium sulfate fractionation, and affinity chromatography on Matrex gel Orange A purified fumarase (EC 4.2.1.2) 632-fold with an 18% yield from crude extracts of Euglena gracilis var. bacillaris. The apparent molecular weight of the native enzyme was 120,000 as determined by gel filtration on Sephacryl S-300. The preparation was over 95% pure, and the subunit molecular weight was 60,000 as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, indicating that the enzyme is a dimer composed of two identical subunits. The pH optimum for E. gracilis fumarase was 8.4. The Km values for malate and fumarate were 1.4 and 0.031 mM, respectively. Preparative two-dimensional gel electrophoresis was used to further purify the enzyme for antibody production. On Ouchterlony double-immunodiffusion gels, the antifumarase serum gave a sharp precipitin line against total E. gracilis protein and purified E. gracilis fumarase. It did not cross-react with purified pig heart fumarase. On immunoblots of purified E. gracilis fumarase and crude cell extracts of E. gracilis, the antibody recognized a single polypeptide with a molecular weight of approximately 60,000, indicating that the antibody is monospecific. This polypeptide was found in E. gracilis mitochondria. The antibody cross-reacted with an Escherichia coli protein whose molecular weight was approximately 60,000, the reported molecular weight of the fumA gene product of E. coli, but it failed to cross-react with proteins found in crude mouse cell extracts, Bacillus subtilis extracts, or purified pig heart fumarase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeckmans S., Kanarek L. A new purification procedure for fumarase based of affinity chromatography. Isolation and characterization of pig-liver fumarase. Eur J Biochem. 1977 Sep;78(2):437–444. doi: 10.1111/j.1432-1033.1977.tb11756.x. [DOI] [PubMed] [Google Scholar]
- Beeckmans S., Kanarek L. Chicken heart fumarase: its purification and physico-chemical characterization. A comparison with the enzyme from pig heart. Int J Biochem. 1982;14(6):453–460. doi: 10.1016/0020-711x(82)90112-4. [DOI] [PubMed] [Google Scholar]
- Beeckmans S., Kanarek L. Demonstration of physical interactions between consecutive enzymes of the citric acid cycle and of the aspartate-malate shuttle. A study involving fumarase, malate dehydrogenase, citrate synthesis and aspartate aminotransferase. Eur J Biochem. 1981 Jul;117(3):527–535. doi: 10.1111/j.1432-1033.1981.tb06369.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chaudhuri S., Thomas E. W. Affinity chromatography of pig heart fumarase. Biochem J. 1979 Jan 1;177(1):115–119. doi: 10.1042/bj1770115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J. R., Roberts R. E. Cloning, mapping, and expression of the fumarase gene of Escherichia coli K-12. J Bacteriol. 1983 Feb;153(2):588–596. doi: 10.1128/jb.153.2.588-596.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D. A simple spectrophotometric assay for fumarate hydratase in crude tissue extracts. Anal Biochem. 1978 Mar;85(1):271–275. doi: 10.1016/0003-2697(78)90299-3. [DOI] [PubMed] [Google Scholar]
- Horrum M. A., Schwartzbach S. D. Induction of fumarase in resting Euglena. Biochim Biophys Acta. 1982 Feb 25;714(3):407–414. doi: 10.1016/0304-4165(82)90147-7. [DOI] [PubMed] [Google Scholar]
- KANAREK L., HILL R. L. THE PREPARATION AND CHARACTERIZATION OF FUMARASE FROM SWINE HEART MUSCLE. J Biol Chem. 1964 Dec;239:4202–4206. [PubMed] [Google Scholar]
- Kobayashi K., Yamanishi T., Tuboi S. Physicochemical, catalytic, and immunochemical properties of fumarases crystallized separately from mitochondrial and cytosolic fractions of rat liver. J Biochem. 1981 Jun;89(6):1923–1931. doi: 10.1093/oxfordjournals.jbchem.a133394. [DOI] [PubMed] [Google Scholar]
- Miles J. S., Guest J. R. Complete nucleotide sequence of the fumarase gene fumA, of Escherichia coli. Nucleic Acids Res. 1984 Apr 25;12(8):3631–3642. doi: 10.1093/nar/12.8.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Penner P. E., Cohen L. H. Effects of adenosine triphosphate and magnesium ions on the fumarase reaction. J Biol Chem. 1969 Feb 10;244(3):1070–1075. [PubMed] [Google Scholar]
- Smith B. R., Hall R. Measurement of thyrotropin receptor antibodies. Methods Enzymol. 1981;74(Pt 100):405–420. doi: 10.1016/0076-6879(81)74029-1. [DOI] [PubMed] [Google Scholar]
- Teipel J. W., Hill R. L. The subunit interactions of fumarase. J Biol Chem. 1971 Aug 10;246(15):4859–4865. [PubMed] [Google Scholar]
- Tokunaga M., Nakano Y., Kitaoka S. Subcellular localization of the GABA-shunt enzymes in Euglena gracilis strain Z. J Protozool. 1979 Aug;26(3):471–473. doi: 10.1111/j.1550-7408.1979.tb04655.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. S., Stedman J. D., West M. H., Pantazis P., Bonner W. M. Discontinuous agarose electrophoretic system for the recovery of stained proteins from polyacrylamide gels. Anal Biochem. 1982 Aug;124(2):264–271. doi: 10.1016/0003-2697(82)90037-9. [DOI] [PubMed] [Google Scholar]
- Yamato S., Murachi T. Dissociation and association of fumarase subunits with special reference to the formation of a functional tetramer. Eur J Biochem. 1979 Jan 2;93(1):189–195. doi: 10.1111/j.1432-1033.1979.tb12810.x. [DOI] [PubMed] [Google Scholar]





