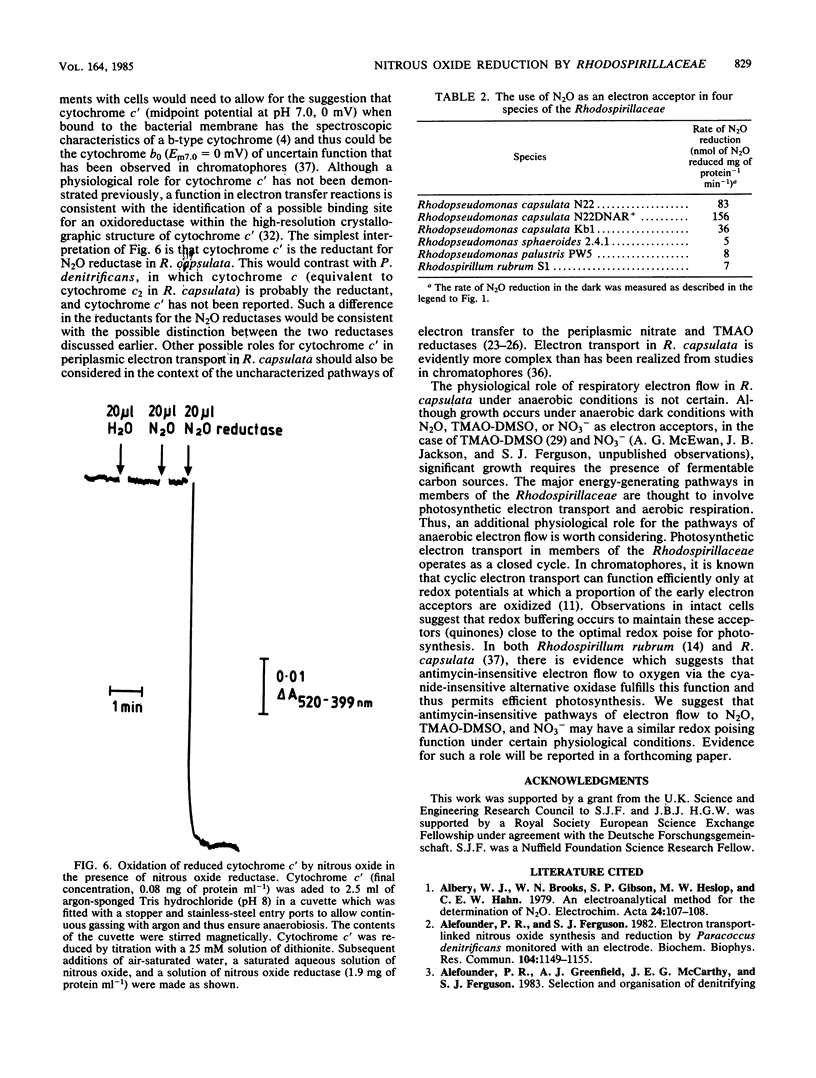
Abstract
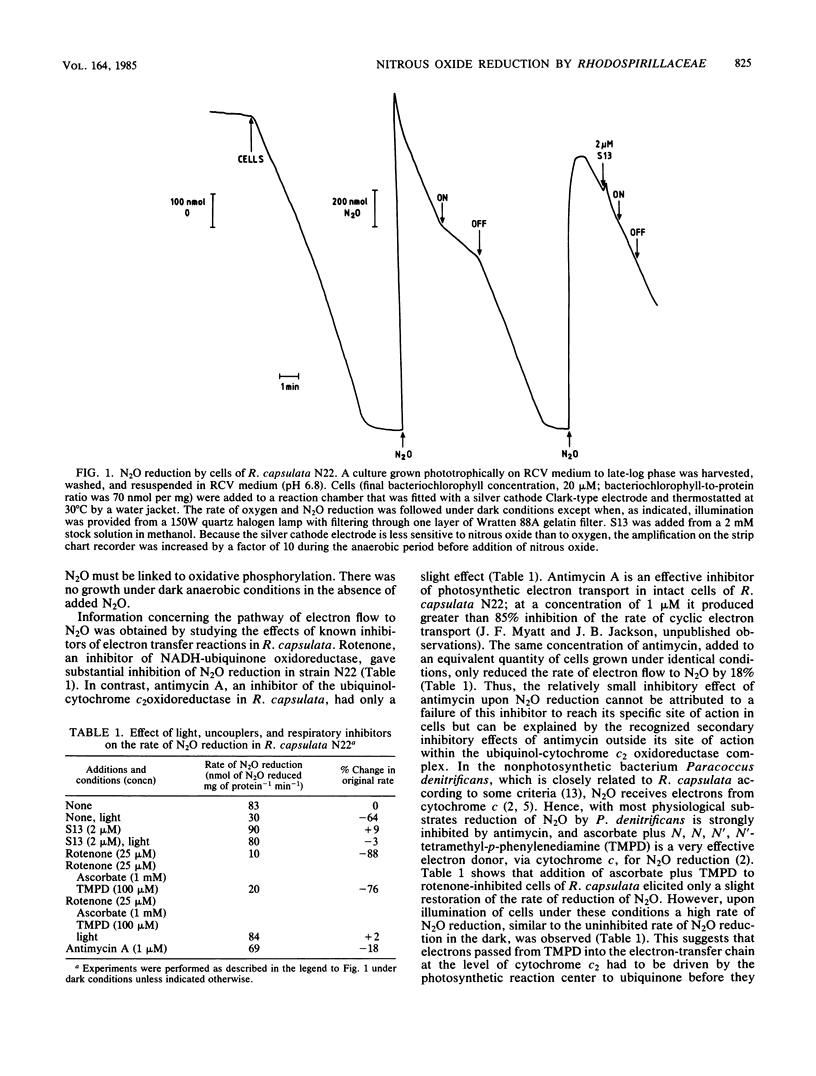
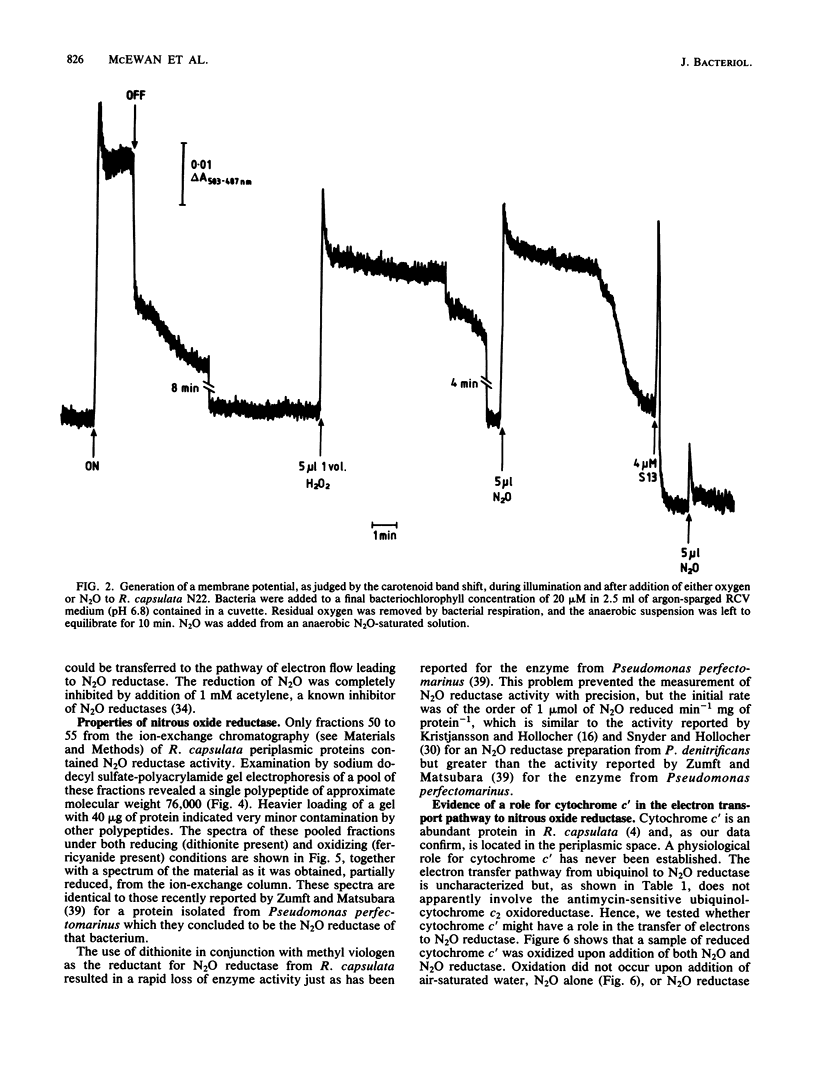
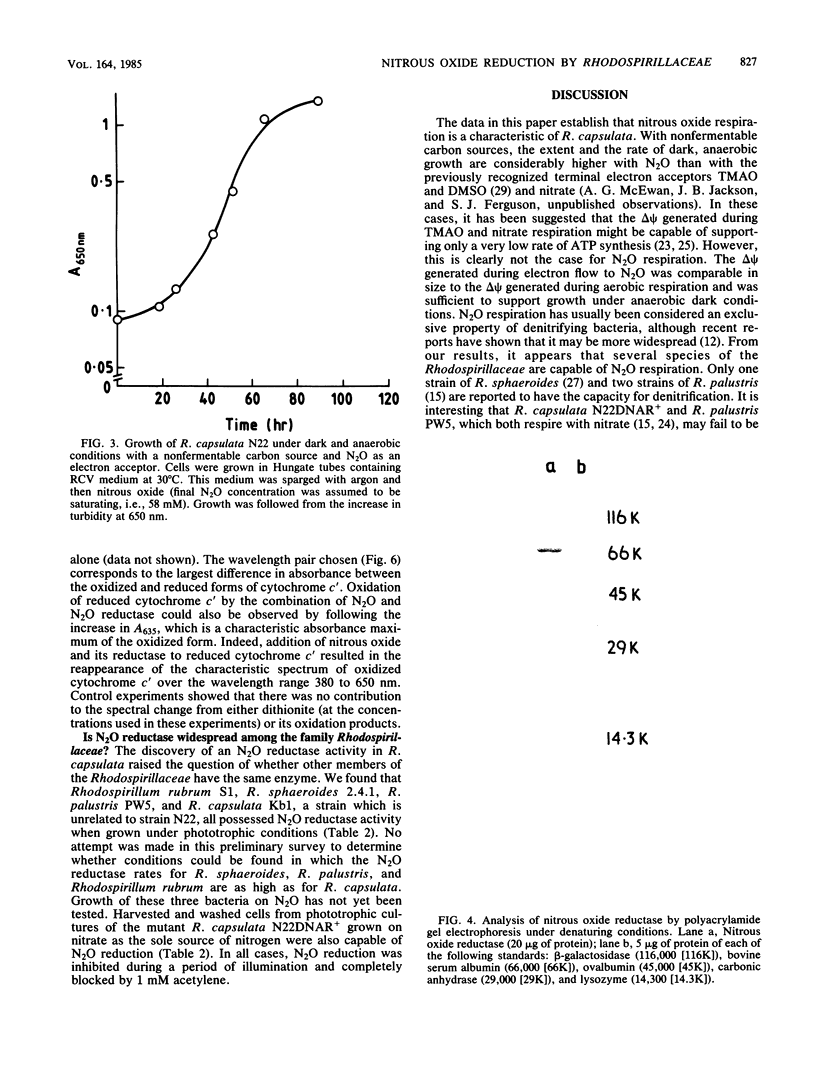
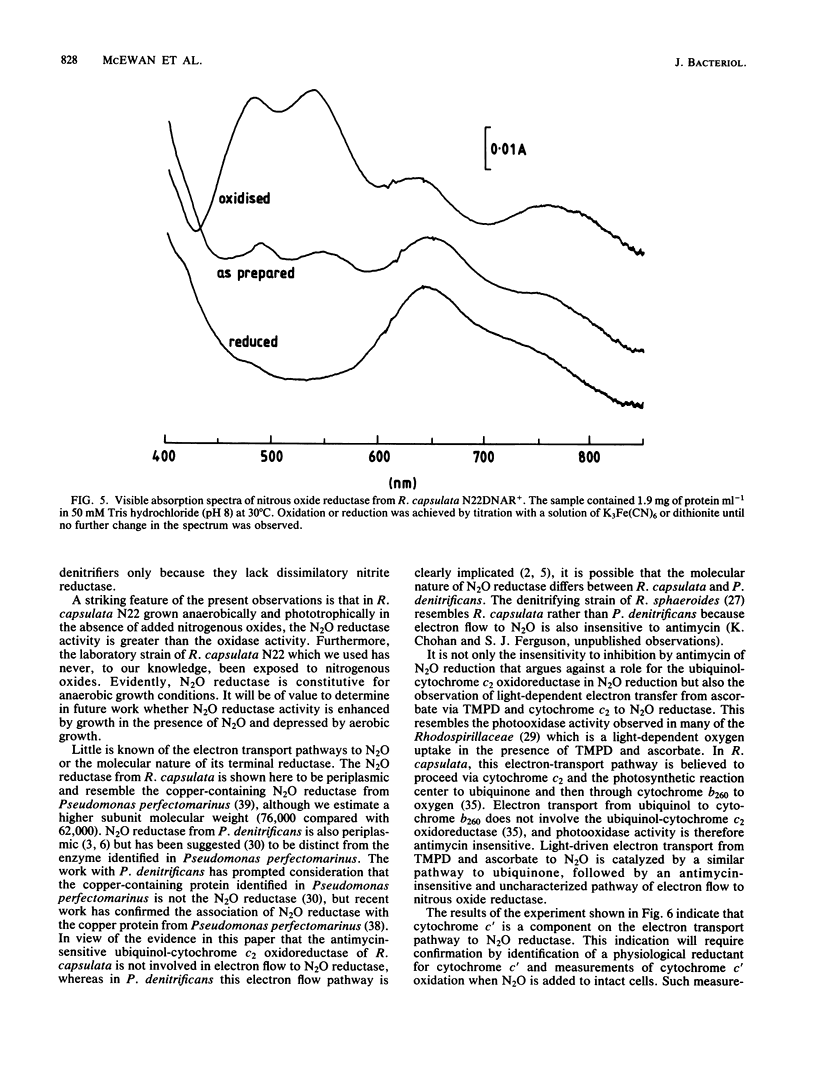
After growth in the absence of nitrogenous oxides under anaerobic phototrophic conditions, several strains of Rhodopseudomonas capsulata were shown to possess a nitrous oxide reductase activity. The enzyme responsible for this activity had a periplasmic location and resembled a nitrous oxide reductase purified from Pseudomonas perfectomarinus. Electron flow to nitrous oxide reductase was coupled to generation of a membrane potential and inhibited by rotenone but not antimycin. It is suggested that electron flow to nitrous oxide reductase branches at the level of ubiquinone from the previously characterized electron transfer components of R. capsulata. This pathway of electron transport could include cytochrome c', a component hitherto without a recognized function. R. capsulata grew under dark anaerobic conditions in the presence of malate as carbon source and nitrous oxide as electron acceptor. This confirms that nitrous oxide respiration is linked to ATP synthesis. Phototrophically and anaerobically grown cultures of nondenitrifying strains of Rhodopseudomonas sphaeroides, Rhodopseudomonas palustris, and Rhodospirillum rubrum also possessed nitrous oxide reductase activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alefounder P. R., Ferguson S. J. Electron transport-linked nitrous oxide synthesis and reduction by Paracoccus denitrificans monitored with an electrode. Biochem Biophys Res Commun. 1982 Feb 11;104(3):1149–1155. doi: 10.1016/0006-291x(82)91370-5. [DOI] [PubMed] [Google Scholar]
- Boogerd F. C., van Verseveld H. W., Stouthamer A. H. Electron transport to nitrous oxide in Paracoccus denitrificans. FEBS Lett. 1980 May 5;113(2):279–284. doi: 10.1016/0014-5793(80)80609-0. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. TOWARD THE ISOLATION OF A PHOTOCHEMICAL REACTION CENTER IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1963 Nov 29;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- Cotton N. P., Clark A. J., Jackson J. B. Interaction between the respiratory and photosynthetic electron transport chains of intact cells of Rhodopseudomonas capsulata mediated by membrane potential. Eur J Biochem. 1983 Feb 15;130(3):581–587. doi: 10.1111/j.1432-1033.1983.tb07189.x. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton P. L., Jackson J. B. Thermodynamic and kinetic characterization of electron transfer components in situ in Rhodopseudomonas spheroides and Rhodospirillum rubrum. Eur J Biochem. 1972 Nov 7;30(3):495–510. doi: 10.1111/j.1432-1033.1972.tb02121.x. [DOI] [PubMed] [Google Scholar]
- Giménez-Gallego G., del Valle-Tascón S., Ramírez J. M. A possible physiological function of the oxygen-photoreducing system of Rhodospirillum rubrum. Arch Microbiol. 1976 Aug;109(1-2):119–125. doi: 10.1007/BF00425123. [DOI] [PubMed] [Google Scholar]
- Kristjansson J. K., Hollocher T. C. First practical assay for soluble nitrous oxide reductase of denitrifying bacteria and a partial kinetic characterization. J Biol Chem. 1980 Jan 25;255(2):704–707. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambin P. Reliability of molecular weight determination of proteins by polyacrylamide gradient gel electrophoresis in the presence of sodium dodecyl sulfate. Anal Biochem. 1978 Mar;85(1):114–125. doi: 10.1016/0003-2697(78)90281-6. [DOI] [PubMed] [Google Scholar]
- Madigan M. T., Gest H. Growth of the photosynthetic bacterium Rhodopseudomonas capsulata chemoautotrophically in darkness with H2 as the energy source. J Bacteriol. 1979 Jan;137(1):524–530. doi: 10.1128/jb.137.1.524-530.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. E., Ferguson S. J. Respiratory control and the basis of light-induced inhibition of respiration in chromatophores from Rhodopseudomonas capsulata. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1406–1411. doi: 10.1016/s0006-291x(82)80155-1. [DOI] [PubMed] [Google Scholar]
- McEwan A. G., Ferguson S. J., Jackson J. B. Electron flow to dimethylsulphoxide or trimethylamine-N-oxide generates a membrane potential in Rhodopseudomonas capsulata. Arch Microbiol. 1983 Dec;136(4):300–305. doi: 10.1007/BF00425221. [DOI] [PubMed] [Google Scholar]
- Satoh T., Hoshino Y., Kitamura H. Rhodopseudomonas sphaeroides forma sp. denitrificans, a denitrifying strain as a subspecies of Rhodopseudomonas sphaeroides. Arch Microbiol. 1976 Jul;108(3):265–269. doi: 10.1007/BF00454851. [DOI] [PubMed] [Google Scholar]
- Schultz J. E., Weaver P. F. Fermentation and anaerobic respiration by Rhodospirillum rubrum and Rhodopseudomonas capsulata. J Bacteriol. 1982 Jan;149(1):181–190. doi: 10.1128/jb.149.1.181-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. W., Hollocher T. C. Nitrous oxide reductase and the 120,000 MW copper protein of N2-producing denitrifying bacteria are different entities. Biochem Biophys Res Commun. 1984 Mar 15;119(2):588–592. doi: 10.1016/s0006-291x(84)80289-2. [DOI] [PubMed] [Google Scholar]
- Weaver P. F., Wall J. D., Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Howard A., Xuong N. H., Salemme F. R. Crystallographic structure of Rhodospirillum molischianum ferricytochrome c' at 2.5 A resolution. J Mol Biol. 1981 Dec 5;153(2):399–424. doi: 10.1016/0022-2836(81)90286-2. [DOI] [PubMed] [Google Scholar]
- Yoshinari T., Knowles R. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem Biophys Res Commun. 1976 Apr 5;69(3):705–710. doi: 10.1016/0006-291x(76)90932-3. [DOI] [PubMed] [Google Scholar]
- Zannoni D., Jasper P., Marrs B. Light-induced oxygen reduction as a probe of electron transport between respiratory and photosynthetic components in membranes of Rhodopseudomonas capsulata. Arch Biochem Biophys. 1978 Dec;191(2):625–631. doi: 10.1016/0003-9861(78)90400-9. [DOI] [PubMed] [Google Scholar]



