Abstract
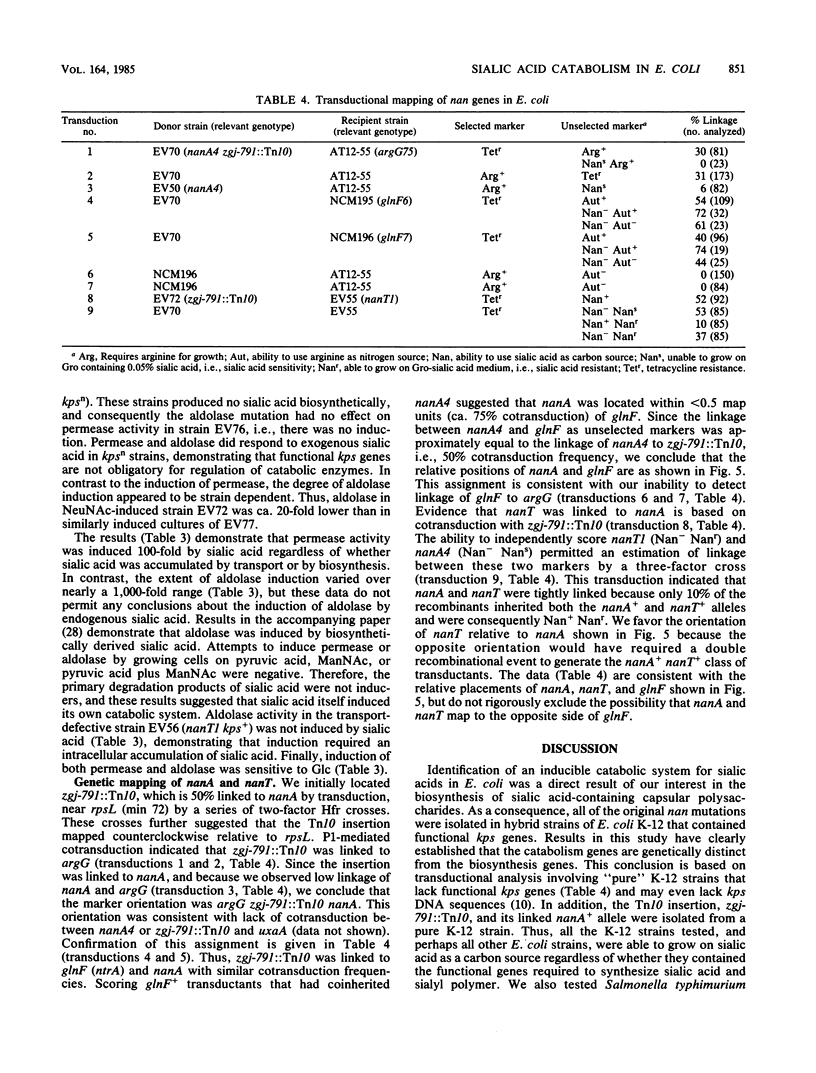
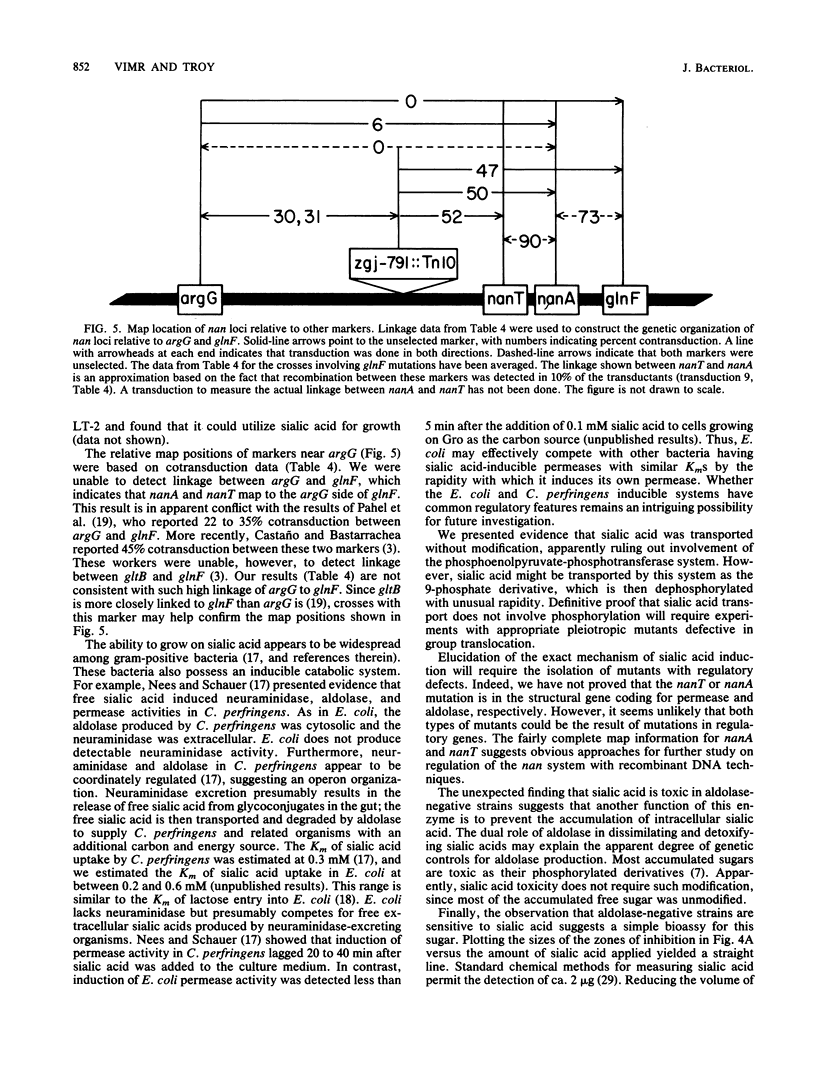
Escherichia coli K-12 and K-12 hybrid strains constructed to express a polysialic acid capsule, the K1 antigen, were able to efficiently use sialic acid as a sole carbon source. This ability was dependent on induction of at least two activities: a sialic acid-specific transport activity, and an aldolase activity specific for cleaving sialic acids. Induction over basal levels required sialic acid as the apparent inducer, and induction of both activities was repressed by glucose. Induction also required the intracellular accumulation of sialic acid, which could be either added exogenously to the medium or accumulated intracellularly through biosynthesis. Exogenous sialic acid appeared to be transported by an active mechanism that did not involve covalent modification of the sugar. Mutations affecting either the transport or degradation of sialic acid prevented its use as a carbon source and have been designated nanT and nanA, respectively. These mutations were located by transduction near min 69 on the E. coli K-12 genetic map, between argG and glnF. In addition to being unable to use sialic acid as a carbon source, aldolase-negative mutants were growth-inhibited by this sugar. Therefore, the intracellularly accumulated sialic acid was toxic in aldolase-deficient E. coli strains. The dual role of aldolase in dissimilating and detoxifying sialic acids is consistent with the apparent multiple controls on expression of this enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUNETTI P., JOURDIAN G. W., ROSEMAN S. The sialic acids. III. Distribution and properties of animal N-acetylneuraminic aldolase. J Biol Chem. 1962 Aug;237:2447–2453. [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMB D. G., ROSEMAN S. The sialic acids. I. The structure and enzymatic synthesis of N-acetylneuraminic acid. J Biol Chem. 1960 Sep;235:2529–2537. [PubMed] [Google Scholar]
- Castaño I., Bastarrachea F. glnF-lacZ fusions in Escherichia coli: studies on glnF expression and its chromosomal orientation. Mol Gen Genet. 1984;195(1-2):228–233. doi: 10.1007/BF00332751. [DOI] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echarti C., Hirschel B., Boulnois G. J., Varley J. M., Waldvogel F., Timmis K. N. Cloning and analysis of the K1 capsule biosynthesis genes of Escherichia coli: lack of homology with Neisseria meningitidis group B DNA sequences. Infect Immun. 1983 Jul;41(1):54–60. doi: 10.1128/iai.41.1.54-60.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Davis M. A., Roberts D. E., Takeshita K., Kleckner N. Genetic organization of transposon Tn10. Cell. 1981 Jan;23(1):201–213. doi: 10.1016/0092-8674(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Jann K., Jann B. The K antigens of Escherichia coli. Prog Allergy. 1983;33:53–79. doi: 10.1159/000407421. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Tolbert N. E., Bieber L. L. Protein determination in membrane and lipoprotein samples: manual and automated procedures. Methods Enzymol. 1981;72:296–303. doi: 10.1016/s0076-6879(81)72018-4. [DOI] [PubMed] [Google Scholar]
- Page M. G., West I. C. Kinetics of lactose transport into Escherichia coli in the presence and absence of a protonmotive force. FEBS Lett. 1980 Nov 3;120(2):187–191. doi: 10.1016/0014-5793(80)80294-8. [DOI] [PubMed] [Google Scholar]
- Pahel G., Zelenetz A. D., Tyler B. M. gltB gene and regulation of nitrogen metabolism by glutamine synthetase in Escherichia coli. J Bacteriol. 1978 Jan;133(1):139–148. doi: 10.1128/jb.133.1.139-148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr T. E., Troy F. A. Structure and biosynthesis of surface polymers containing polysialic acid in Escherichia coli. J Biol Chem. 1980 Mar 25;255(6):2332–2342. [PubMed] [Google Scholar]
- Schauer R., Wember M., Wirtz-Peitz F., Ferreira do Amaral C. Studies on the substrate specificity of acylneuraminate pyruvate-lyase. Hoppe Seylers Z Physiol Chem. 1971 Aug;352(8):1073–1080. doi: 10.1515/bchm2.1971.352.2.1073. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Hoopes B. C., McClure W. R., Kleckner N. Three promoters near the termini of IS10: pIN, pOUT, and pIII. Cell. 1983 Sep;34(2):673–682. doi: 10.1016/0092-8674(83)90400-2. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Troy F. A., 2nd The chemistry and biosynthesis of selected bacterial capsular polymers. Annu Rev Microbiol. 1979;33:519–560. doi: 10.1146/annurev.mi.33.100179.002511. [DOI] [PubMed] [Google Scholar]
- Troy F. A., McCloskey M. A. Role of a membranous sialyltransferase complex in the synthesis of surface polymers containing polysialic acid in Escherichia coli. Temperature-induced alteration in the assembly process. J Biol Chem. 1979 Aug 10;254(15):7377–7387. [PubMed] [Google Scholar]
- Vimr E. R., McCoy R. D., Vollger H. F., Wilkison N. C., Troy F. A. Use of prokaryotic-derived probes to identify poly(sialic acid) in neonatal neuronal membranes. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1971–1975. doi: 10.1073/pnas.81.7.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., Miller C. G. Dipeptidyl carboxypeptidase-deficient mutants of Salmonella typhimurium. J Bacteriol. 1983 Mar;153(3):1252–1258. doi: 10.1128/jb.153.3.1252-1258.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., Troy F. A. Regulation of sialic acid metabolism in Escherichia coli: role of N-acylneuraminate pyruvate-lyase. J Bacteriol. 1985 Nov;164(2):854–860. doi: 10.1128/jb.164.2.854-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]