Abstract
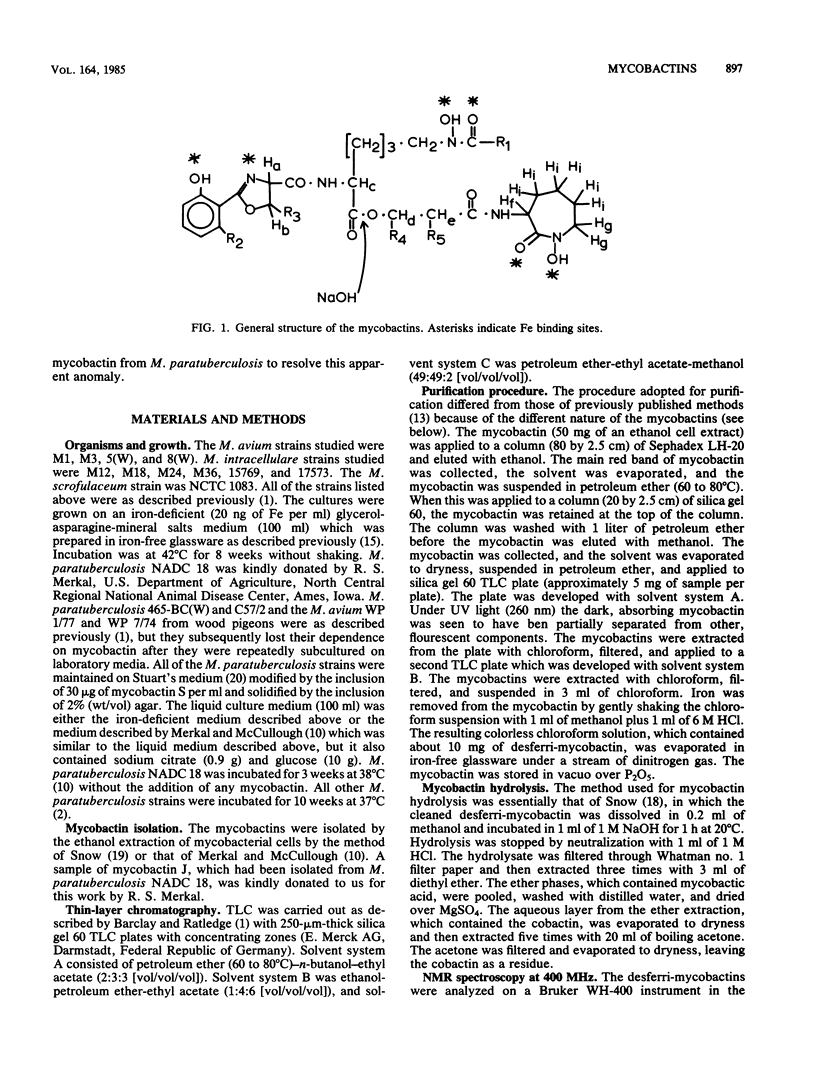
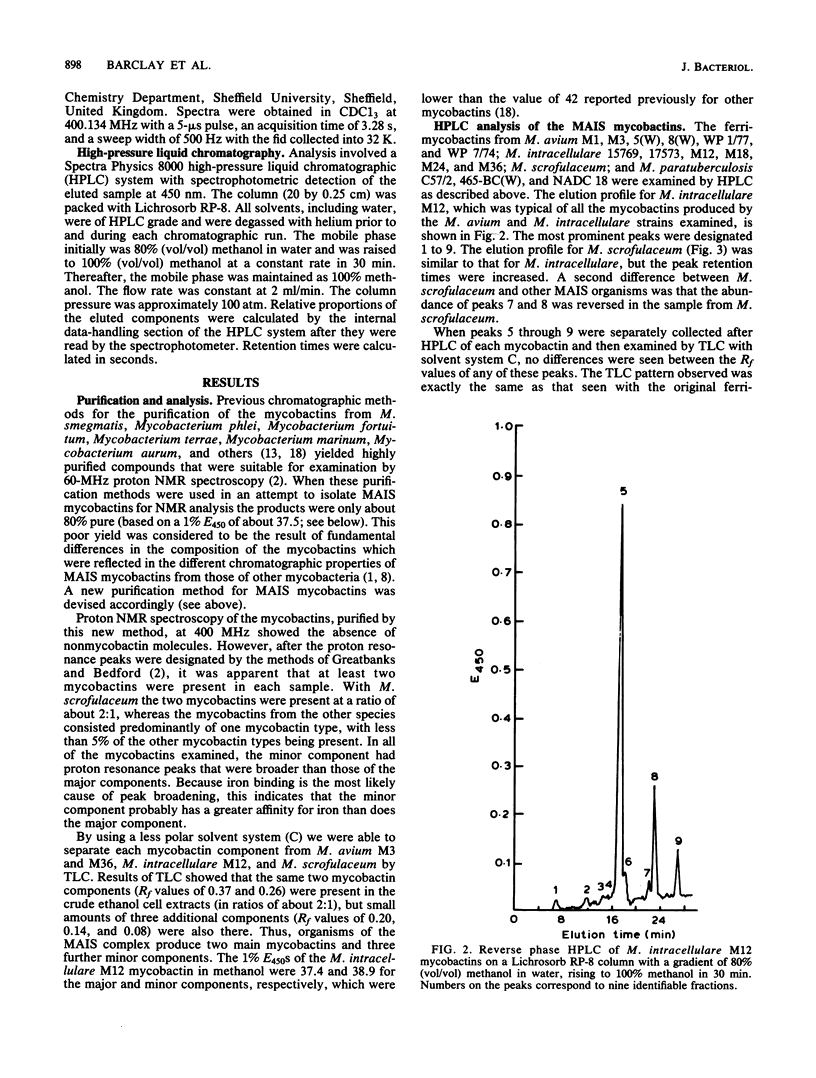
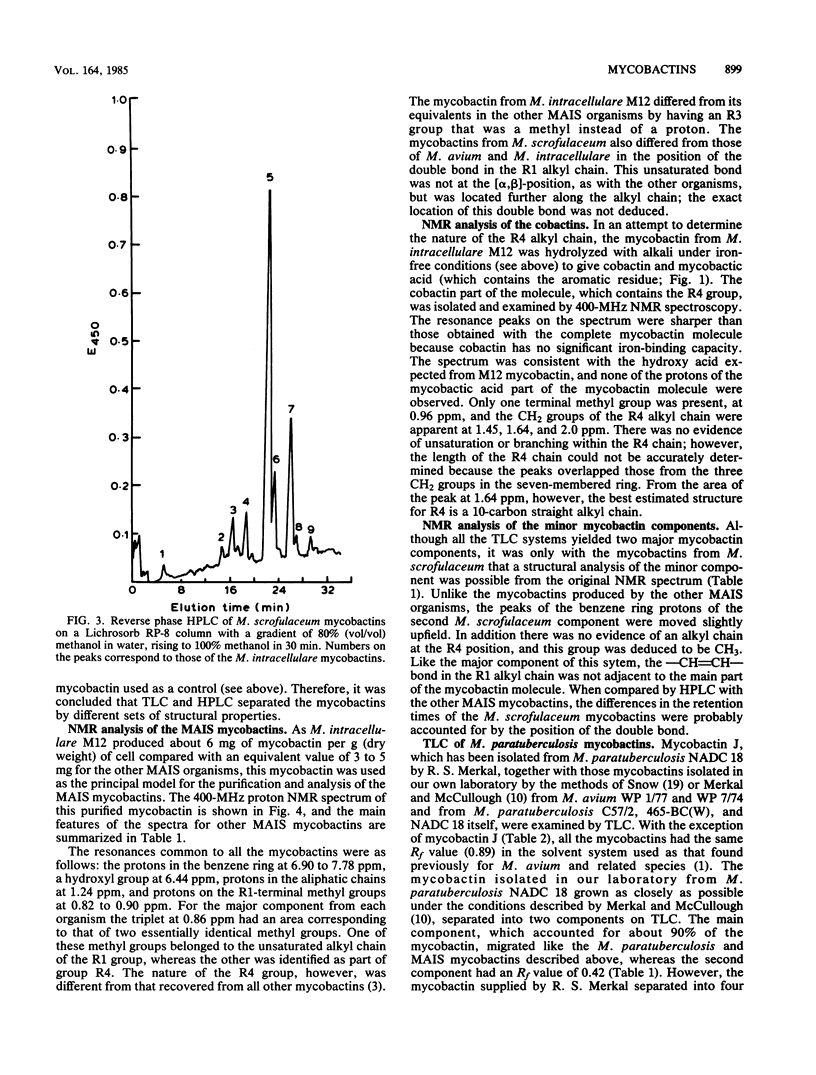
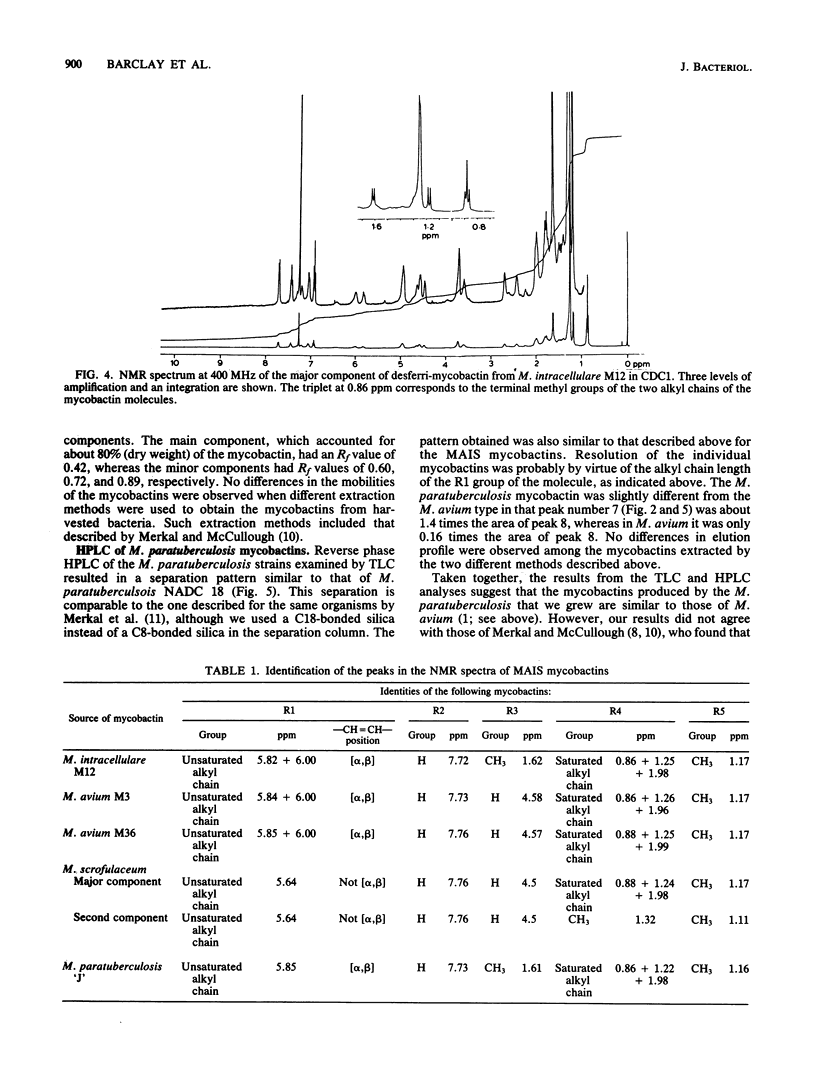
Methods were devised to purify the cell-associated, iron-binding compounds known as mycobactins from the closely related species Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum (i.e., the MAIS complex of organisms). The mycobactins from these three species showed a structure that is common to the mycobactins from all the mycobacteria examined to date. However, these mycobactins were unique in that they had more than one alkyl chain. The M. scrofulaceum mycobactins differed from other MAIS mycobactins by a shift in the position of the double bond in the R1 alkyl chain. Traces of other mycobactin types were observed in ethanol extracts of the three species, and examination of the chromatographic properties of these mycobactins showed that each species produced five mycobactin types. Each mycobactin could be subdivided further by the length of its R1 alkyl chain. No differences in the production of these novel mycobactin were observed among species. Mycobactins from three strains of Mycobacterium paratuberculosis and two wood pigeon strains of Mycobacterium avium which had lost their original growth requirements for mycobactin after repeated subculturing in laboratory growth media were examined by thin-layer chromatography and high-pressure liquid chromatography. Each organism produced a mycobactin with similar chromatographic properties to those synthesized by MAIS organisms. M. paratuberculosis NADC 18 produced at least two components in our laboratory, and nuclear magnetic resonance analysis of the major component showed this mycobactin to be identical to that produced by M. intracellulare M12. However, a sample of mycobactin J isolated by Merkal and McCullough (Curr. Microbiol. 7:333-335, 1982) from M. paratuberculosis NADC 18 was different from our isolates and appeared to correspond to a minor mycobactin component we had seen by thin-layer chromatography. No reason for this difference could be evinced. Our findings indicate that there is a close taxonomic relationship between M. paratuberculosis and the MAIS complex.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay R., Ratledge C. Iron-binding compounds of Mycobacterium avium, M. intracellulare, M. scrofulaceum, and mycobactin-dependent M. paratuberculosis and M. avium. J Bacteriol. 1983 Mar;153(3):1138–1146. doi: 10.1128/jb.153.3.1138-1146.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greatbanks D., Bedford G. R. Identification of mycobactins by nuclear-magnetic-resonance spectroscopy. Biochem J. 1969 Dec;115(5):1047–1050. doi: 10.1042/bj1151047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. M., Ratledge C. Mycobactins as chemotaxonomic characters for some rapidly growing mycobacteria. J Gen Microbiol. 1984 Aug;130(8):1883–1892. doi: 10.1099/00221287-130-8-1883. [DOI] [PubMed] [Google Scholar]
- Hough E., Rogers D. The crystal structure of ferrimycobactin P, a growth factor for the Mycobacteria. Biochem Biophys Res Commun. 1974 Mar 15;57(1):73–77. doi: 10.1016/s0006-291x(74)80358-x. [DOI] [PubMed] [Google Scholar]
- Matthews P. R., McDiarmid A., Collins P., Brown A. The dependence of some strains of Mycobacterium avium on mycobactin for initial and subsequent growth. J Med Microbiol. 1978 Feb;11(1):53–57. doi: 10.1099/00222615-11-1-53. [DOI] [PubMed] [Google Scholar]
- Merkal R. S., Curran B. J. Growth and metabolic characteristics of Mycobacterium paratuberculosis. Appl Microbiol. 1974 Aug;28(2):276–279. doi: 10.1128/am.28.2.276-279.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C., Ewing D. F. The separation of the mycobactins from Mycobacterium smegmatis by using high-pressure liquid chromatography. Biochem J. 1978 Dec 1;175(3):853–857. doi: 10.1042/bj1750853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C., Hall M. J. Influence of metal ions on the formation of mycobactin and salicylic acid in Mycobacterium smegmatis grown in static culture. J Bacteriol. 1971 Oct;108(1):314–319. doi: 10.1128/jb.108.1.314-319.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C., Patel P. V., Mundy J. Iron transport in Mycobacterium smegmatis: the location of mycobactin by electron microscopy. J Gen Microbiol. 1982 Jul;128(7):1559–1565. doi: 10.1099/00221287-128-7-1559. [DOI] [PubMed] [Google Scholar]
- STUART P. VACCINATION AGAINST JOHNE'S DISEASE IN CATTLE EXPOSED TO EXPERIMENTAL INFECTION. Br Vet J. 1965 Jul;121:289–318. doi: 10.1016/s0007-1935(17)41102-x. [DOI] [PubMed] [Google Scholar]
- Snow G. A. Isolation and structure of mycobactin T, a growth factor from Mycobacterium tuberculosis. Biochem J. 1965 Oct;97(1):166–175. doi: 10.1042/bj0970166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow G. A. Mycobactins: iron-chelating growth factors from mycobacteria. Bacteriol Rev. 1970 Jun;34(2):99–125. doi: 10.1128/br.34.2.99-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel M. F., Valette L. Etude de quelques souches de Mycobacterium paratuberculosis: caractéres biochimiques et activité allergique. Ann Rech Vet. 1976;7(2):207–213. [PubMed] [Google Scholar]


