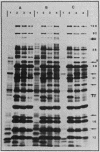
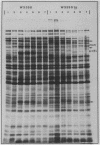
Abstract
A new mutation in the htpR (rpoH) gene of Escherichia coli K-12 was identified. The mutation resulted in a temperature-sensitive phenotype in terms of cell growth and bacteriophage lambda development. As in the case of the classical htpR tsn-165 mutation, synthesis of heat shock polypeptides was not induced in strains carrying the mutation described here.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A., Gold M. Isolation of the bacteriophage lambda A-gene protein. Proc Natl Acad Sci U S A. 1975 Feb;72(2):581–585. doi: 10.1073/pnas.72.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL A. Sensitive mutants of bacteriophage lambda. Virology. 1961 May;14:22–32. doi: 10.1016/0042-6822(61)90128-3. [DOI] [PubMed] [Google Scholar]
- Cooper S., Ruettinger T. A temperature sensitive nonsense mutation affecting the synthesis of a major protein of Escherichia coli K12. Mol Gen Genet. 1975 Aug 5;139(2):167–176. doi: 10.1007/BF00264696. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. R., Georgopoulos C., Tilly K., Herskowitz I., Banuett F. Interactions of bacteriophage and host macromolecules in the growth of bacteriophage lambda. Microbiol Rev. 1984 Dec;48(4):299–325. doi: 10.1128/mr.48.4.299-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst C. R., Bingham H., Bouchard J. P. Temperature sensitivity in Escherichia coli K12: mutants unable to support normal growth of lambda phage at high temperatures. Virology. 1978 Jun 15;87(2):416–436. doi: 10.1016/0042-6822(78)90145-9. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Erickson J. W., Gross C. A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984 Sep;38(2):383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Zhou Y. N., Gross C., Heilig J., Christie G. E., Calendar R. Mutations in the rpoH (htpR) gene of Escherichia coli K-12 phenotypically suppress a temperature-sensitive mutant defective in the sigma 70 subunit of RNA polymerase. J Bacteriol. 1985 Mar;161(3):939–943. doi: 10.1128/jb.161.3.939-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landick R., Vaughn V., Lau E. T., VanBogelen R. A., Erickson J. W., Neidhardt F. C. Nucleotide sequence of the heat shock regulatory gene of E. coli suggests its protein product may be a transcription factor. Cell. 1984 Aug;38(1):175–182. doi: 10.1016/0092-8674(84)90538-5. [DOI] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Lau E. T. Molecular cloning and expression of a gene that controls the high-temperature regulon of Escherichia coli. J Bacteriol. 1983 Feb;153(2):597–603. doi: 10.1128/jb.153.2.597-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A. Positive regulatory gene for temperature-controlled proteins in Escherichia coli. Biochem Biophys Res Commun. 1981 May 29;100(2):894–900. doi: 10.1016/s0006-291x(81)80257-4. [DOI] [PubMed] [Google Scholar]
- Oxender D. L., Anderson J. J., Daniels C. J., Landick R., Gunsalus R. P., Zurawski G., Selker E., Yanofsky C. Structural and functional analysis of cloned DNA containing genes responsible for branched-chain amino acid transport in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1412–1416. doi: 10.1073/pnas.77.3.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner-Smith M., Becker A. DNA packaging in the lambdoid phages: identification of the products of phi 80 genes 1 and 2. Virology. 1981 Jun;111(2):629–641. doi: 10.1016/0042-6822(81)90362-7. [DOI] [PubMed] [Google Scholar]
- Tobe T., Ito K., Yura T. Isolation and physical mapping of temperature-sensitive mutants defective in heat-shock induction of proteins in Escherichia coli. Mol Gen Genet. 1984;195(1-2):10–16. doi: 10.1007/BF00332716. [DOI] [PubMed] [Google Scholar]
- Waghorne C., Fuerst C. R. Involvement of the htpR gene product of Escherichia coli in phage lambda development. Virology. 1985 Feb;141(1):51–64. doi: 10.1016/0042-6822(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Genetic control of heat-shock protein synthesis and its bearing on growth and thermal resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 Feb;79(3):860–864. doi: 10.1073/pnas.79.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]