Abstract
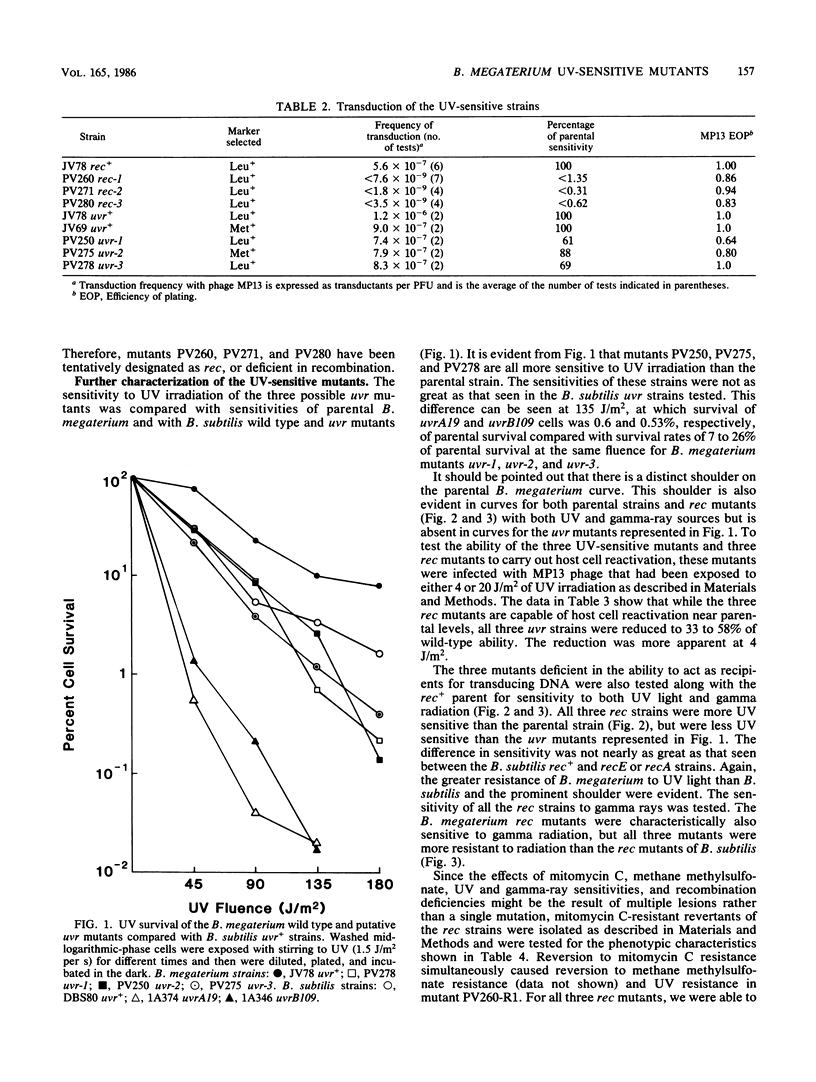
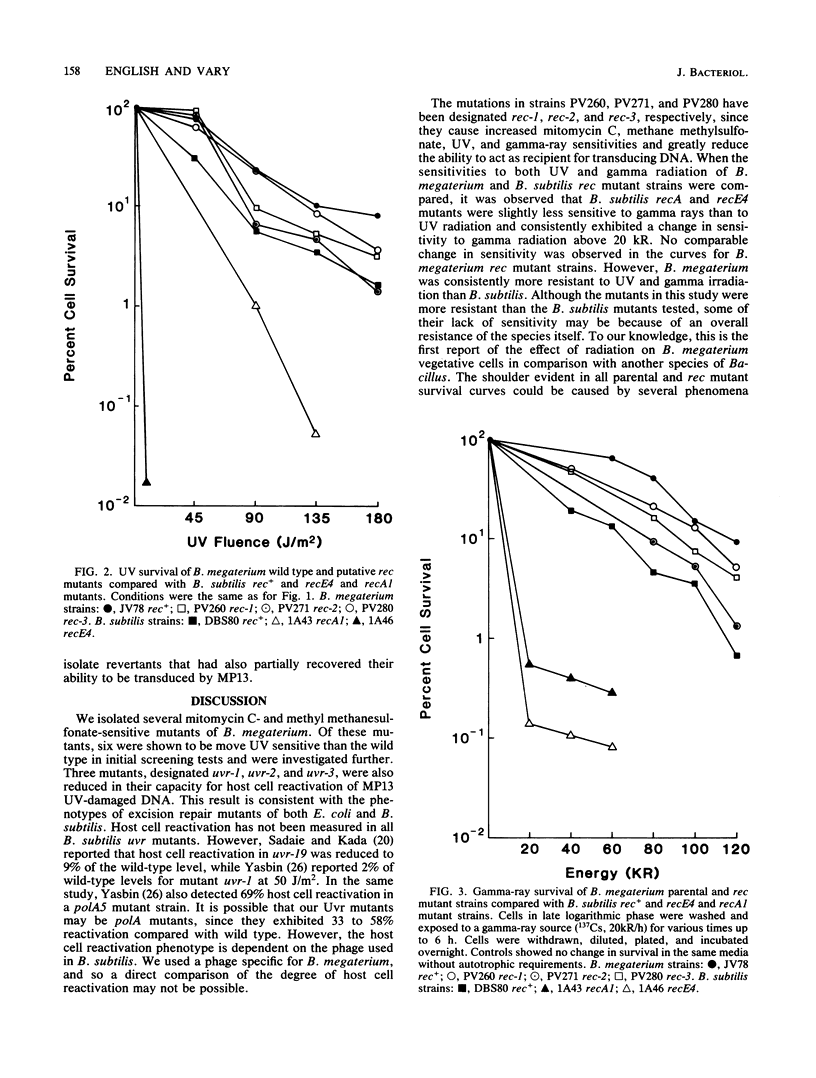
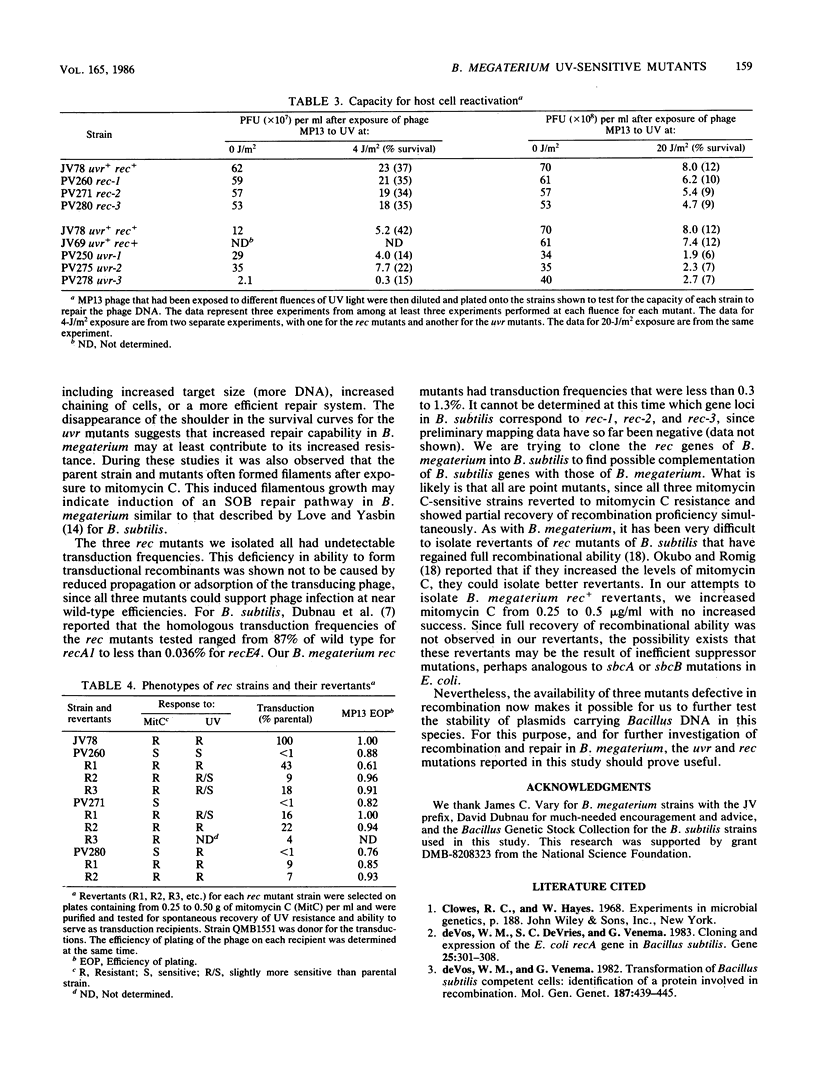
Mutants of Bacillus megaterium QMB1551 sensitive to mitomycin C or methyl methanesulfonate were isolated and characterized phenotypically. Cell survival after UV-light and gamma-ray exposure was determined, as was transductional recombination. Of the mutants tested, three were sensitive to UV but remained recombination proficient. The UV-sensitive mutants were also reduced in host cell reactivation. At least three mutants had undetectable transduction frequencies, i.e., less than 0.3 to 1.3% of the parental strain frequencies, and so appear to be recombination deficient. Sensitivities of these mutant strains to UV light and gamma radiation were compared with those of parental B. megaterium as well as parental, recE4, recA1, uvrA19, and uvrB109 strains of Bacillus subtilis. In each case, the strains of B. megaterium, including the parental strains, showed a higher percentage of cell survival than B. subtilis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dodson L. A., Hadden C. T. Capacity for postreplication repair correlated with transducibility in Rec- mutants of Bacillus subtilis. J Bacteriol. 1980 Nov;144(2):608–615. doi: 10.1128/jb.144.2.608-615.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Genetic characterization of recombination-deficient mutants of Bacillus subtilis. J Bacteriol. 1974 Feb;117(2):488–493. doi: 10.1128/jb.117.2.488-493.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Scher B., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: phenotypic characterization of radiation-sensitive recombination-deficient mutants. J Bacteriol. 1973 Apr;114(1):273–286. doi: 10.1128/jb.114.1.273-286.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitner G., Manteuffel R., Hofemeister J. Functional substitution of the recE gene of Bacillus subtilis by the recA gene of Proteus mirabilis. Mol Gen Genet. 1984;195(3):516–522. doi: 10.1007/BF00341456. [DOI] [PubMed] [Google Scholar]
- Friedman B. M., Yasbin R. E. The genetics and specificity of the constitutive excision repair system of Bacillus subtilis. Mol Gen Genet. 1983;190(3):481–486. doi: 10.1007/BF00331080. [DOI] [PubMed] [Google Scholar]
- Hoch J. A., Anagnostopoulos C. Chromosomal location and properties of radiation sensitivity mutations in Bacillus subtilis. J Bacteriol. 1970 Aug;103(2):295–301. doi: 10.1128/jb.103.2.295-301.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Love P. E., Yasbin R. E. Genetic characterization of the inducible SOS-like system of Bacillus subtilis. J Bacteriol. 1984 Dec;160(3):910–920. doi: 10.1128/jb.160.3.910-920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza G., Fortunato A., Ferrari E., Canosi U., Falaschi A., Polsinelli M. Genetic and enzymic studies on the recombination process in Bacillus subtilis. Mol Gen Genet. 1975;136(1):9–30. doi: 10.1007/BF00275445. [DOI] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Impaired transformability of Bacillus subtilis mutant sensitive to mitomycin C and ultraviolet radiation. J Mol Biol. 1966 Feb;15(2):440–454. doi: 10.1016/s0022-2836(66)80120-1. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., Hoch J. A. Revised genetic linkage map of Bacillus subtilis. Microbiol Rev. 1985 Jun;49(2):158–179. doi: 10.1128/mr.49.2.158-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaie Y., Kada T. Recombination-deficient mutants of Bacillus subtilis. J Bacteriol. 1976 Feb;125(2):489–500. doi: 10.1128/jb.125.2.489-500.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp R. J., Bown K. J., Atkinson A. Phenotypic and genotypic characterization of some thermophilic species of Bacillus. J Gen Microbiol. 1980 Mar;117(1):201–210. doi: 10.1099/00221287-117-1-201. [DOI] [PubMed] [Google Scholar]
- Vary P. S., Garbe J. C., Franzen M., Frampton E. W. MP13, a generalized transducing bacteriophage for Bacillus megaterium. J Bacteriol. 1982 Mar;149(3):1112–1119. doi: 10.1128/jb.149.3.1112-1119.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger S., Evenchick Z., Hertman I. Postincision steps of photoproduct removal in a mutant of Bacillus cereus 569 that produces UV-sensitive spores. J Bacteriol. 1983 Nov;156(2):909–913. doi: 10.1128/jb.156.2.909-913.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E. DNA repair in Bacillus subtilis. I. The presence of an inducible system. Mol Gen Genet. 1977 Jun 8;153(2):211–218. [PubMed] [Google Scholar]
- de Vos W. M., Venema G. Transformation of Bacillus subtilis competent cells: identification of a protein involved in recombination. Mol Gen Genet. 1982;187(3):439–445. doi: 10.1007/BF00332625. [DOI] [PubMed] [Google Scholar]
- de Vos W. M., de Vries S. C., Venema G. Cloning and expression of the Escherichia coli recA gene in Bacillus subtilis. Gene. 1983 Nov;25(2-3):301–308. doi: 10.1016/0378-1119(83)90234-2. [DOI] [PubMed] [Google Scholar]


