Abstract
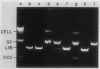
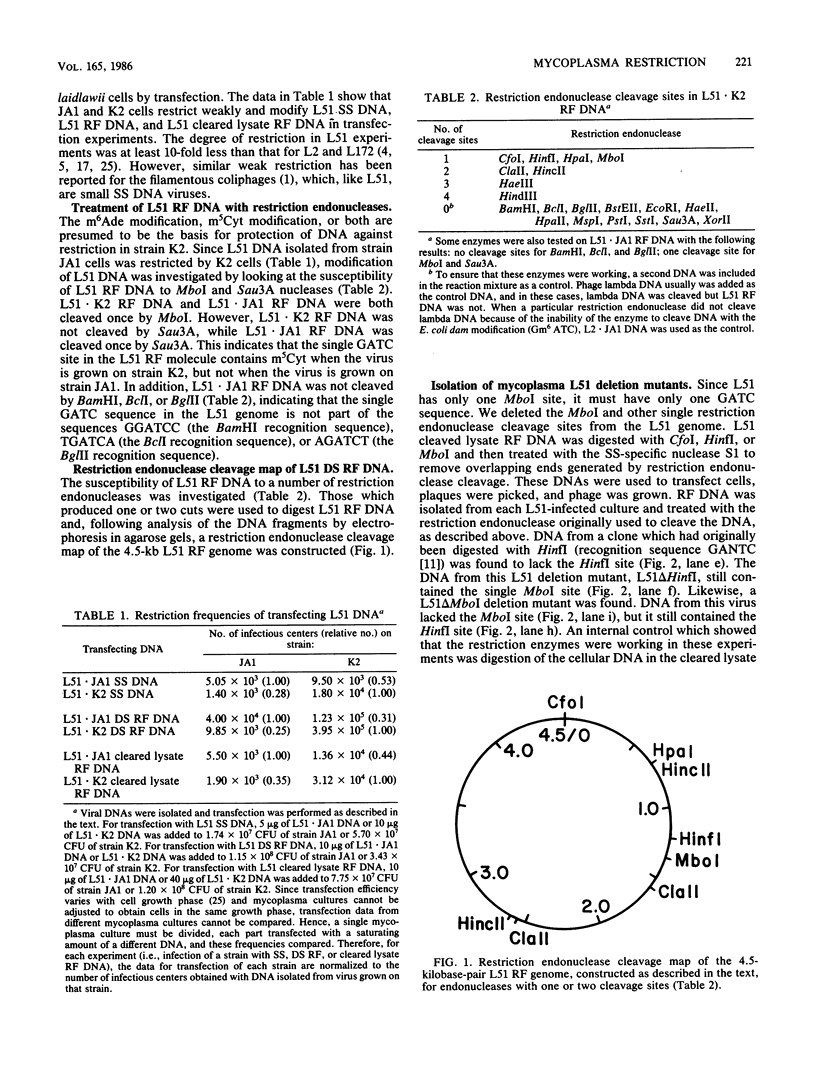
Mycoplasma bacteriophage L51 single-stranded DNA and L2 double-stranded DNA are host cell modified and restricted when they transfect Acholeplasma laidlawii JA1 and K2 cells. The L51 genome has a single restriction endonuclease MboI site (recognition sequence GATC), which contains 5-methylcytosine when the DNA is isolated from L51 phage grown in K2 cells but is unmethylated when the DNA is from phage grown in JA1 cells. This GATC sequence is nonessential, since an L51 mutant in which the MboI site was deleted was still viable. DNA from this deletion mutant phage was not restricted during transfection of either strain K2 or JA1. Therefore, strain K2 restricts DNA containing the sequence GATC, and strain JA1 restricts DNA containing the sequence GAT 5-methylcytosine. We conclude that K2 cells have a restriction system specific for DNA containing the sequence GATC and protect their DNA by methylating cytosine in this sequence. In contrast, JA1 cells (which contain no methylated DNA bases) have a newly discovered type of restriction-modification system. From results of studies of the restriction of specifically methylated DNAs, we conclude that JA1 cells restrict DNA containing 5-methylcytosine, regardless of the nucleotide sequence containing 5-methylcytosine. This is the first report of a DNA restriction activity specific for a single (methylated) base. Modification in this system is the absence of cytosine methylating activity. A restriction-deficient variant of strain JA1, which retains the JA1 modification phenotype, was isolated, indicating that JA1 cells have a gene product with restriction specificity for DNA containing 5-methylcytosine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W. Host specificity of DNA produced by Escherichia coli. 9. Host-controlled modification of bacteriophage fd. J Mol Biol. 1966 Oct;20(3):483–496. doi: 10.1016/0022-2836(66)90004-0. [DOI] [PubMed] [Google Scholar]
- Brooks J. E., Roberts R. J. Modification profiles of bacterial genomes. Nucleic Acids Res. 1982 Feb 11;10(3):913–934. doi: 10.1093/nar/10.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J., Gourlay R. N., Hull R., Garwes D. J. Ultrastructure of Mycoplasmatales virus laidlawii I. J Gen Virol. 1972 Aug;16(2):215–221. doi: 10.1099/0022-1317-16-2-215. [DOI] [PubMed] [Google Scholar]
- Dybvig K., Nowak J. A., Sladek T. L., Maniloff J. Identification of an enveloped phage, mycoplasma virus L172, that contains a 14-kilobase single-stranded DNA genome. J Virol. 1985 Feb;53(2):384–390. doi: 10.1128/jvi.53.2.384-390.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K., Swinton D., Maniloff J., Hattman S. Cytosine methylation of the sequence GATC in a mycoplasma. J Bacteriol. 1982 Sep;151(3):1420–1424. doi: 10.1128/jb.151.3.1420-1424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Das J., Maniloff J. Lack of repair of ultraviolet light damage in Mycoplasma gallisepticum. J Mol Biol. 1977 Oct 25;116(2):337–344. doi: 10.1016/0022-2836(77)90221-2. [DOI] [PubMed] [Google Scholar]
- Gourlay R. N. Mycoplasmatales virus-laidlawii 2, a new virus isolated from Acholeplasma laidlawii. J Gen Virol. 1971 Jul;12(1):65–67. doi: 10.1099/0022-1317-12-1-65. [DOI] [PubMed] [Google Scholar]
- Gourlay R. N., Wyld S. G. Isolation of mycoplasmatales virus-laidlawii 3, a new virus infecting Acholeplasma laidlawii. J Gen Virol. 1973 May;19(2):279–283. doi: 10.1099/0022-1317-19-2-279. [DOI] [PubMed] [Google Scholar]
- Haberer K., Klotz G., Maniloff J., Kleinschmidt A. K. Structural and biological properties of mycoplasmavirus MVL3: an unusual virus-procaryote interaction. J Virol. 1979 Oct;32(1):268–275. doi: 10.1128/jvi.32.1.268-275.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler C., Neumaier P. S., Wolf W. Recognition sequences of restriction endonucleases and methylases--a review. Gene. 1985;33(1):1–102. doi: 10.1016/0378-1119(85)90119-2. [DOI] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Complementary specificity of restriction endonucleases of Diplococcus pneumoniae with respect to DNA methylation. J Mol Biol. 1977 Jul;114(1):153–168. doi: 10.1016/0022-2836(77)90289-3. [DOI] [PubMed] [Google Scholar]
- Liss A., Maniloff J. Infection of Acholeplasma laidlawii by MVL51 virus. Virology. 1973 Sep;55(1):118–126. doi: 10.1016/s0042-6822(73)81013-x. [DOI] [PubMed] [Google Scholar]
- Liss A., Maniloff J. Isolation of Mycoplasmatales viruses and characterization of MVL1, MVL52, and MVG51. Science. 1971 Aug 20;173(3998):725–727. doi: 10.1126/science.173.3998.725. [DOI] [PubMed] [Google Scholar]
- Maniloff J. Evolution of wall-less prokaryotes. Annu Rev Microbiol. 1983;37:477–499. doi: 10.1146/annurev.mi.37.100183.002401. [DOI] [PubMed] [Google Scholar]
- Maniloff J., Haberer K., Gourlay R. N., Das J., Cole R. Mycoplasma viruses. Intervirology. 1982;18(4):177–188. doi: 10.1159/000149323. [DOI] [PubMed] [Google Scholar]
- Maniloff J., Morowitz H. J. Cell biology of the mycoplasmas. Bacteriol Rev. 1972 Sep;36(3):263–290. doi: 10.1128/br.36.3.263-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M., Christ C., Schildkraut I. Alteration of apparent restriction endonuclease recognition specificities by DNA methylases. Nucleic Acids Res. 1984 Jul 11;12(13):5165–5173. doi: 10.1093/nar/12.13.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak J. A., Das J., Maniloff J. Characterization of an Acholeplasma laidlawii variant with a REP- phenotype. J Bacteriol. 1976 Aug;127(2):832–836. doi: 10.1128/jb.127.2.832-836.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddar S. K., Cadden S. P., Das J., Maniloff J. Heterogeneous progeny viruses are produced by a budding enveloped phage. Intervirology. 1985;23(4):208–221. doi: 10.1159/000149607. [DOI] [PubMed] [Google Scholar]
- Radman M., Wagner R. Effects of DNA methylation on mismatch repair, mutagenesis, and recombination in Escherichia coli. Curr Top Microbiol Immunol. 1984;108:23–28. doi: 10.1007/978-3-642-69370-0_3. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction and modification enzymes and their recognition sequences. Nucleic Acids Res. 1984;12 (Suppl):r167–r204. doi: 10.1093/nar/12.suppl.r167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek T. L., Maniloff J. Polyethylene glycol-dependent transfection of Acholeplasma laidlawii with mycoplasma virus L2 DNA. J Bacteriol. 1983 Aug;155(2):734–741. doi: 10.1128/jb.155.2.734-741.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri B., Nagaraja V., Bickle T. A. Bacterial DNA modification. Curr Top Microbiol Immunol. 1984;108:1–9. doi: 10.1007/978-3-642-69370-0_1. [DOI] [PubMed] [Google Scholar]
- Walder R. Y., Walder J. A., Donelson J. E. The organization and complete nucleotide sequence of the PstI restriction-modification system. J Biol Chem. 1984 Jun 25;259(12):8015–8026. [PubMed] [Google Scholar]
- Wells R. D., Goodman T. C., Hillen W., Horn G. T., Klein R. D., Larson J. E., Müller U. R., Neuendorf S. K., Panayotatos N., Stirdivant S. M. DNA structure and gene regulation. Prog Nucleic Acid Res Mol Biol. 1980;24:167–267. doi: 10.1016/s0079-6603(08)60674-1. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Maniloff J., Zablen L. B. Phylogenetic analysis of the mycoplasmas. Proc Natl Acad Sci U S A. 1980 Jan;77(1):494–498. doi: 10.1073/pnas.77.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]