Abstract
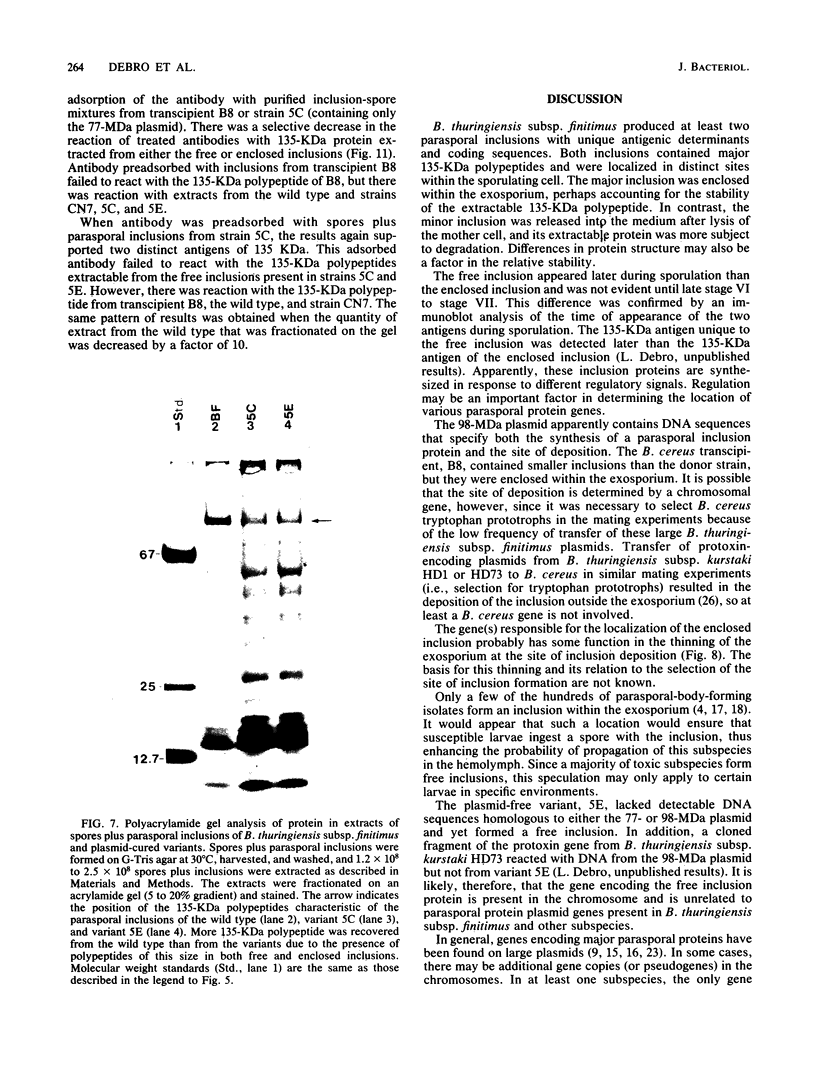
Bacillus thuringiensis subsp. finitimus produced at least two parasporal inclusions. One inclusion was formed within the exosporium and remained with the spore after mother cell lysis. A second inclusion formed somewhat later exterior to the exosporium. Each inclusion contained a major polypeptide of about 135,000 daltons with unique antigenic determinants. This subspecies contained only two plasmids, of 98 and 77 megadaltons (MDa). Strains cured of these plasmids produced only the free inclusion. Since the plasmid-cured strains did not contain DNA sequences homologous to plasmid DNA, the gene for the free-inclusion protein must be encoded in the chromosome. In contrast, the enclosed parasporal inclusion was produced only when the plasmid of 98 MDa was present. In addition, transfer of the 98-MDa plasmid to Bacillus cereus resulted in transcipients that produced small inclusions enclosed within the exosporium, and the protein extracted from these inclusions reacted with antibody specific for enclosed inclusion protein of B. thuringiensis subsp. finitimus. Genes in both the chromosome and a plasmid function in the synthesis of distinct parasporal proteins in this subspecies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Angelo N., Holt S. C. Regulation of extracellular protease production in Bacillus cereus T: characterization of mutants producing altered amounts of protease. J Bacteriol. 1971 Jun;106(3):1016–1025. doi: 10.1128/jb.106.3.1016-1025.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. Structure and morphogenesis of the bacterial spore coat. Bacteriol Rev. 1976 Jun;40(2):360–402. doi: 10.1128/br.40.2.360-402.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulla L. A., Jr, Bechtel D. B., Kramer K. J., Shethna Y. I., Aronson A. I., Fitz-James P. C. Ultrastructure, physiology, and biochemistry of Bacillus thuringiensis. Crit Rev Microbiol. 1980;8(2):147–204. doi: 10.3109/10408418009081124. [DOI] [PubMed] [Google Scholar]
- Bulla L. A., Jr, Davidson L. I., Kramer K. J., Jones B. L. Purification of the insecticidal toxin from the parasporal crystal of Bacillus thuringiensis subsp. kurstaki. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1123–1130. doi: 10.1016/0006-291x(79)91997-1. [DOI] [PubMed] [Google Scholar]
- Bulla L. A., Jr, Kramer K. J., Davidson L. I. Characterization of the entomocidal parasporal crystal of Bacillus thuringiensis. J Bacteriol. 1977 Apr;130(1):375–383. doi: 10.1128/jb.130.1.375-383.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Fitz-James P. C. Formation of protoplasts from resting spores. J Bacteriol. 1971 Mar;105(3):1119–1136. doi: 10.1128/jb.105.3.1119-1136.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González J. M., Jr, Brown B. J., Carlton B. C. Transfer of Bacillus thuringiensis plasmids coding for delta-endotoxin among strains of B. thuringiensis and B. cereus. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6951–6955. doi: 10.1073/pnas.79.22.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González J. M., Jr, Carlton B. C. Patterns of plasmid DNA in crystalliferous and acrystalliferous strains of Bacillus thuringiensis. Plasmid. 1980 Jan;3(1):92–98. doi: 10.1016/s0147-619x(80)90038-4. [DOI] [PubMed] [Google Scholar]
- HANNAY C. L. Fowler's bacillus and its parasporal body. J Biophys Biochem Cytol. 1961 Feb;9:285–298. doi: 10.1083/jcb.9.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIMPEL A. M., ANGUS T. A. The taxonomy of insect pathogens related to Bacillus cereus Frankland and Frankland. Can J Microbiol. 1958 Oct;4(5):531–541. doi: 10.1139/m58-058. [DOI] [PubMed] [Google Scholar]
- Held G. A., Bulla L. A., Jr, Ferrari E., Hoch J., Aronson A. I., Minnich S. A. Cloning and localization of the lepidopteran protoxin gene of Bacillus thuringiensis subsp. kurstaki. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6065–6069. doi: 10.1073/pnas.79.19.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klier A., Fargette F., Ribier J., Rapoport G. Cloning and expression of the crystal protein genes from Bacillus thuringiensis strain berliner 1715. EMBO J. 1982;1(7):791–799. doi: 10.1002/j.1460-2075.1982.tb01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad J. W., Schnepf H. E., Whiteley H. R. Diversity of locations for Bacillus thuringiensis crystal protein genes. J Bacteriol. 1983 Apr;154(1):419–428. doi: 10.1128/jb.154.1.419-428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lereclus D., Menou G., Lecadet M. M. Isolation of a DNA sequence related to several plasmids from Bacillus thuringiensis after a mating involving the Streptococcus faecalis plasmid pAM beta 1. Mol Gen Genet. 1983;191(2):307–313. doi: 10.1007/BF00334831. [DOI] [PubMed] [Google Scholar]
- Minnich S. A., Aronson A. I. Regulation of protoxin synthesis in Bacillus thuringiensis. J Bacteriol. 1984 May;158(2):447–454. doi: 10.1128/jb.158.2.447-454.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson tkw, bulla L. A., Jr Lipid metabolism during bacterial growth, sporulation, and germination: an obligate nutritional requirement in Bacillus thuringiensis for compounds that stimulate fatty acid synthesis. J Bacteriol. 1975 Aug;123(2):598–603. doi: 10.1128/jb.123.2.598-603.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepf H. E., Whiteley H. R. Cloning and expression of the Bacillus thuringiensis crystal protein gene in Escherichia coli. Proc Natl Acad Sci U S A. 1981 May;78(5):2893–2897. doi: 10.1073/pnas.78.5.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. A., Walker P. D., Thomson R. O., Somerville H. J. The fine structure of Bacillus finitimus and Bacillus thuringiensis spores with special reference to the location of crystal antigen. J Gen Microbiol. 1974 Oct;84(2):261–276. doi: 10.1099/00221287-84-2-261. [DOI] [PubMed] [Google Scholar]
- Somerville H. J. Formation of the parasporal inclusion of Bacillus thuringiensis. Eur J Biochem. 1971 Jan;18(2):226–237. doi: 10.1111/j.1432-1033.1971.tb01235.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrell D. J., Bulla L. A., Jr, Andrews R. E., Jr, Kramer K. J., Davidson L. I., Nordin P. Comparative biochemistry of entomocidal parasporal crystals of selected Bacillus thuringiensis strains. J Bacteriol. 1981 Feb;145(2):1052–1062. doi: 10.1128/jb.145.2.1052-1062.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG I. E., FITZ-JAMES P. C. Chemical and morphological studies of bacterial spore formation. II. Spore and parasporal protein formation in Bacillus cereus var. alesti. J Biophys Biochem Cytol. 1959 Dec;6:483–498. doi: 10.1083/jcb.6.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]