Abstract
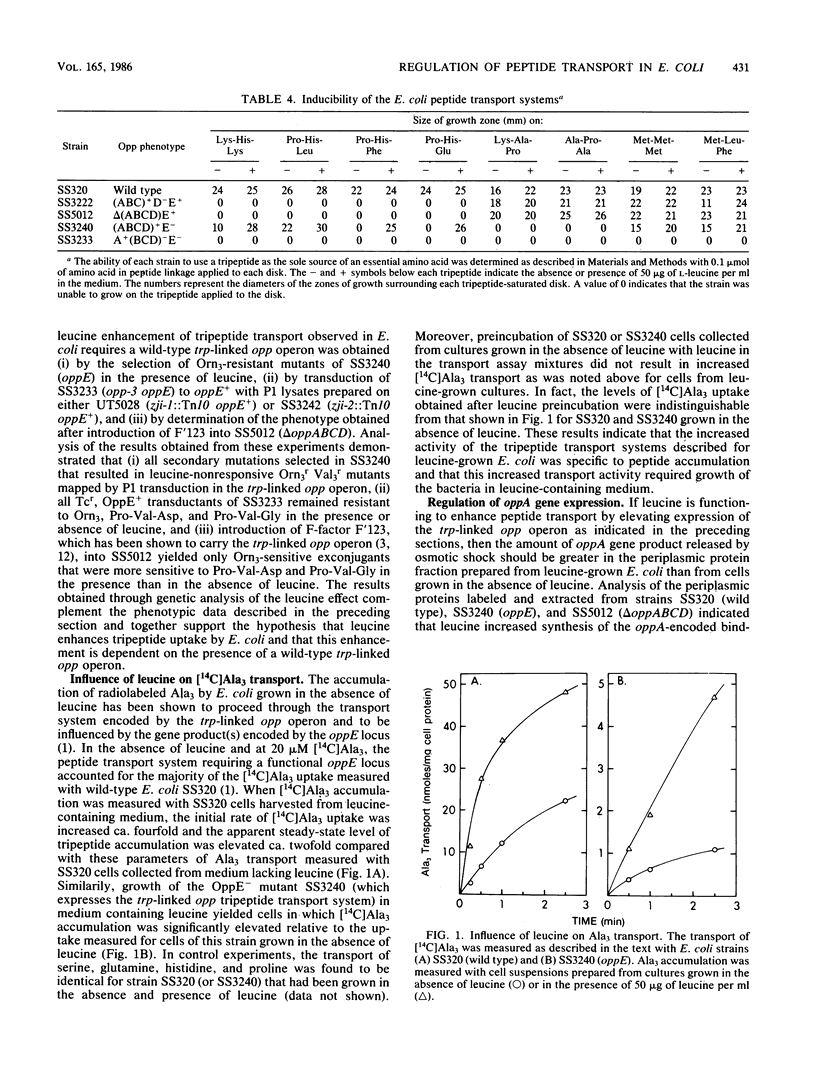
Growth of Escherichia coli in medium containing leucine results in increased entry of exogenously supplied tripeptides into the bacterial cell. This leucine-mediated elevation of peptide transport required expression of the trp-linked opp operon and was accompanied by increased sensitivity to toxic tripeptides, by an enhanced capacity to utilize nutritional peptides, and by an increase in both the velocity and apparent steady-state level of L-[U-14C]alanyl-L-alanyl-L-alanine accumulation for E. coli grown in leucine-containing medium relative to these parameters of peptide transport measured with bacteria grown in media lacking leucine. Direct measurement of opp operon expression by pulse-labeling experiments demonstrated that growth of E. coli in the presence of leucine resulted in increased synthesis of the oppA-encoded periplasmic binding protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J. C., Short S. A. Genetic analysis of Escherichia coli oligopeptide transport mutants. J Bacteriol. 1985 Feb;161(2):484–492. doi: 10.1128/jb.161.2.484-492.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J. C., Short S. A. opp-lac Operon fusions and transcriptional regulation of the Escherichia coli trp-linked oligopeptide permease. J Bacteriol. 1986 Feb;165(2):434–442. doi: 10.1128/jb.165.2.434-442.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Z., Gilvarg C. Triornithine-resistant strains of Escherichia coli. Isolation, definition, and genetic studies. J Biol Chem. 1974 Jan 10;249(1):143–148. [PubMed] [Google Scholar]
- Emr S. D., Bassford P. J., Jr Localization and processing of outer membrane and periplasmic proteins in Escherichia coli strains harboring export-specific suppressor mutations. J Biol Chem. 1982 May 25;257(10):5852–5860. [PubMed] [Google Scholar]
- Higgins C. F., Hardie M. M., Jamieson D., Powell L. M. Genetic map of the opp (Oligopeptide permease) locus of Salmonella typhimurium. J Bacteriol. 1983 Feb;153(2):830–836. doi: 10.1128/jb.153.2.830-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Hardie M. M. Periplasmic protein associated with the oligopeptide permeases of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1983 Sep;155(3):1434–1438. doi: 10.1128/jb.155.3.1434-1438.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth B. G., Higgins C. F. Genetic organization of the oligopeptide permease (opp) locus of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1983 Mar;153(3):1548–1551. doi: 10.1128/jb.153.3.1548-1551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Anaerobic and leucine-dependent expression of a peptide transport gene in Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):131–136. doi: 10.1128/jb.160.1.131-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli strain K-12. J Bacteriol. 1962 Mar;83:624–630. doi: 10.1128/jb.83.3.624-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenny A. B., Margolin P. Locations of the opp and supX genes of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1980 Aug;143(2):747–752. doi: 10.1128/jb.143.2.747-752.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L., Anderson J. J., Daniels C. J., Landick R., Gunsalus R. P., Zurawski G., Selker E., Yanofsky C. Structural and functional analysis of cloned DNA containing genes responsible for branched-chain amino acid transport in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1412–1416. doi: 10.1073/pnas.77.3.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L., Anderson J. J., Mayo M. M., Quay S. C. Leucine binding protein and regulation of transport in E. coli. J Supramol Struct. 1977;6(3):419–431. doi: 10.1002/jss.400060315. [DOI] [PubMed] [Google Scholar]
- Penrose W. R., Nichoalds G. E., Piperno J. R., Oxender D. L. Purification and properties of a leucine-binding protein from Escherichia coli. J Biol Chem. 1968 Nov 25;243(22):5921–5928. [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino acid transport systems in Escherichia coli K-12. J Biol Chem. 1968 Nov 25;243(22):5914–5920. [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]



