Abstract
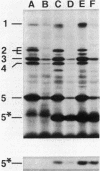
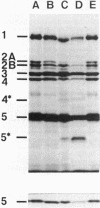
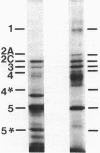
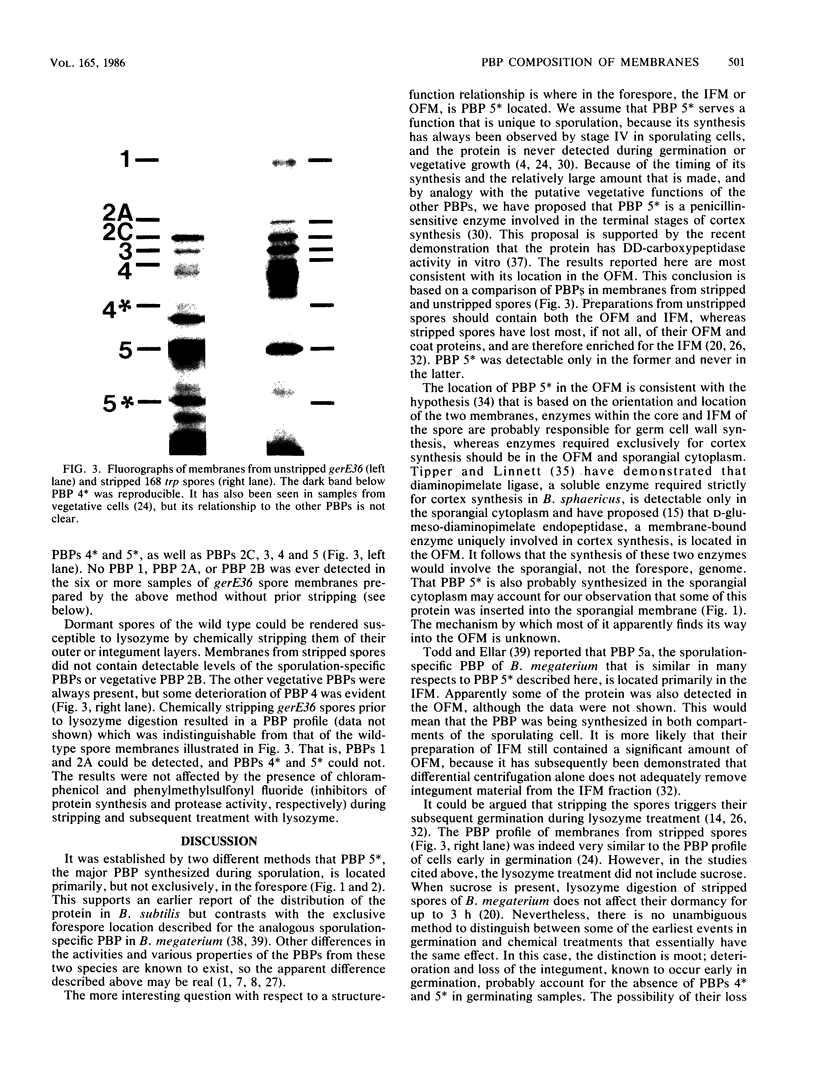
The distribution of penicillin-binding proteins (PBPs) within different membranes of sporulating cells of Bacillus subtilis was examined in an effort to correlate the location of individual PBPs with their proposed involvement in either cortical or vegetative peptidoglycan synthesis. The PBP composition of forespores was determined by two methods: examination of isolated forespore membranes and assay of the in vivo accessibility of the PBPs to penicillin. In both cases, it was apparent that PBP 5*, the major PBP synthesized during sporulation, was present primarily, but not exclusively, in the forespore. The membranes from mature dormant spores were prepared by either chemically stripping the integument layers of the spores, followed by lysozyme digestion, or lysozyme digestion alone of coat-defective gerE spores. PBP 5* was detected in membranes from unstripped spores but was never found in stripped ones, which suggests that the primary location of this PBP is the outer forespore membrane. This is consistent with a role for PBP 5* exclusively in cortex synthesis. In contrast, vegetative PBPs 1 and 2A were only observed in stripped spore preparations that were greatly enriched for the inner forespore membrane, which supports the proposed requirement for these PBPs early in germination. The apparent presence of PBP 3 in both membranes of the spore reinforces the suggestion that it catalyzes a step common to both cortical and vegetative peptidoglycan synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M., Strominger J. L. Five penicillin-binding components occur in Bacillus subtilis membranes. J Biol Chem. 1972 Dec 25;247(24):8107–8113. [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E. Altered membrane proteins in a minicell-producing mutant of Bacillus subtilis. J Bacteriol. 1979 Jul;139(1):305–307. doi: 10.1128/jb.139.1.305-307.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E. Induction of penicillin-binding proteins under catabolite-repressed conditions. J Bacteriol. 1985 Jun;162(3):1302–1303. doi: 10.1128/jb.162.3.1302-1303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Sowell M. O. Stability and synthesis of the penicillin-binding proteins during sporulation. J Bacteriol. 1983 Nov;156(2):545–551. doi: 10.1128/jb.156.2.545-551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Strominger J. L. Altered penicillin-binding components in penicillin-resistant mutants of Bacillus subtilis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1816–1820. doi: 10.1073/pnas.73.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase H. A., Shepherd S. T., Reynolds P. E. Studies on the penicillin-binding components of Bacillus megaterium. FEBS Lett. 1977 Apr 15;76(2):199–203. doi: 10.1016/0014-5793(77)80151-8. [DOI] [PubMed] [Google Scholar]
- Crafts-Lighty A., Ellar D. J. The structure and function of the spore outer membrane in dormant and germinating spores of Bacillus megaterium. J Appl Bacteriol. 1980 Feb;48(1):135–145. doi: 10.1111/j.1365-2672.1980.tb05215.x. [DOI] [PubMed] [Google Scholar]
- Dancer B. N. Requirement for peptidoglycan synthesis during sporulation of Bacillus subtilis. J Bacteriol. 1979 Dec;140(3):786–797. doi: 10.1128/jb.140.3.786-797.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C. Membrane protein alterations during the early stages of sporulation of Bacillus subtilis. J Supramol Struct. 1976;5(4):457–473. doi: 10.1002/jss.400050405. [DOI] [PubMed] [Google Scholar]
- Goldman R. C. Protein composition of cell and forespore membranes of Bacillus subtilis. J Supramol Struct. 1973;1(3):185–193. doi: 10.1002/jss.400010304. [DOI] [PubMed] [Google Scholar]
- Guinand M., Michel G., Tipper D. J. Appearance of gamma-D-glutamyl-(L) meso-diaminopimealate peptidoglycan hydrolase during sporulation in Bacillus sphaericus. J Bacteriol. 1974 Oct;120(1):173–184. doi: 10.1128/jb.120.1.173-184.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. E., Lawrence P. J. Accessibility of the (14C)benzylpenicillin binding proteins in membranes of sporulating bacilli. Antimicrob Agents Chemother. 1975 Jul;8(1):38–44. doi: 10.1128/aac.8.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson G. E., Strominger J. L. Synthesis of peptidoglycan by high molecular weight penicillin-binding proteins of Bacillus subtilis and Bacillus stearothermophilus. J Biol Chem. 1984 Feb 10;259(3):1483–1490. [PubMed] [Google Scholar]
- Jenkinson H. F. Altered arrangement of proteins in the spore coat of a germination mutant of Bacillus subtilis. J Gen Microbiol. 1983 Jun;129(6):1945–1958. doi: 10.1099/00221287-129-6-1945. [DOI] [PubMed] [Google Scholar]
- Kleppe G., Strominger J. L. Studies of the high molecular weight penicillin-binding proteins of Bacillus subtilis. J Biol Chem. 1979 Jun 10;254(11):4856–4862. [PubMed] [Google Scholar]
- Koshikawa T., Beaman T. C., Pankratz H. S., Nakashio S., Corner T. R., Gerhardt P. Resistance, germination, and permeability correlates of Bacillus megaterium spores successively divested of integument layers. J Bacteriol. 1984 Aug;159(2):624–632. doi: 10.1128/jb.159.2.624-632.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. J. Penicillin: reversible inhibition of forespore septum development in Bacillus megaterium cells. Antimicrob Agents Chemother. 1974 Dec;6(6):815–820. doi: 10.1128/aac.6.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Moir A. Germination properties of a spore coat-defective mutant of Bacillus subtilis. J Bacteriol. 1981 Jun;146(3):1106–1116. doi: 10.1128/jb.146.3.1106-1116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyman S. L., Buchanan C. E. Restoration of vegetative penicillin-binding proteins during germination and outgrowth of Bacillus subtilis spores: relationship of individual proteins to specific cell cycle events. J Bacteriol. 1985 Jan;161(1):164–168. doi: 10.1128/jb.161.1.164-168.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice G. A., Wolfe F. H., Clegg L. F. The use of density gradient centrifugation for the separation of germinated from nongerminated spores. J Appl Bacteriol. 1972 Jun;35(2):345–349. doi: 10.1111/j.1365-2672.1972.tb03706.x. [DOI] [PubMed] [Google Scholar]
- Racine F. M., Vary J. C. Isolation and properties of membranes from Bacillus megaterium spores. J Bacteriol. 1980 Sep;143(3):1208–1214. doi: 10.1128/jb.143.3.1208-1214.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. E., Shepherd S. T., Chase H. A. Identification of the binding protein which may be the target of penicillin action in Bacillus megaterium. Nature. 1978 Feb 9;271(5645):568–570. doi: 10.1038/271568a0. [DOI] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell M. O., Buchanan C. E. Changes in penicillin-binding proteins during sporulation of Bacillus subtilis. J Bacteriol. 1983 Mar;153(3):1331–1337. doi: 10.1128/jb.153.3.1331-1337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Kornberg A. Biochemical studies of bacterial sporulation and germination. VI. Origin of spore core and coat proteins. J Biol Chem. 1968 Sep 10;243(17):4588–4599. [PubMed] [Google Scholar]
- Swerdlow R. D., Setlow P. Isolation and characterization of two distinct fractions from the inner membrane of dormant Bacillus megaterium spores. J Bacteriol. 1984 Apr;158(1):9–15. doi: 10.1128/jb.158.1.9-15.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir H., Gilvarg C. Density gradient centrifugation for the separation of sporulating forms of bacteria. J Biol Chem. 1966 Mar 10;241(5):1085–1090. [PubMed] [Google Scholar]
- Tipper D. J., Linnett P. E. Distribution of peptidoglycan synthetase activities between sporangia and forespores in sporulating cells of Bacillus sphaericus. J Bacteriol. 1976 Apr;126(1):213–221. doi: 10.1128/jb.126.1.213-221.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Bone E. J., Ellar D. J. The sporulation-specific penicillin-binding protein 5a from Bacillus subtilis is a DD-carboxypeptidase in vitro. Biochem J. 1985 Sep 15;230(3):825–828. doi: 10.1042/bj2300825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Ellar D. J. Alteration in the penicillin-binding profile of Bacillus megaterium during sporulation. Nature. 1982 Dec 16;300(5893):640–643. doi: 10.1038/300640a0. [DOI] [PubMed] [Google Scholar]
- VINTER V. SPORES OF MICROORGANISMS. XIV. LATE STAGES OF INTRASPORANGIAL DEVELOPMENT OF BACTERIAL SPORES: THEIR SENSITIVITY TO ANTIBIOTICS. Folia Microbiol (Praha) 1964 Mar;18:58–72. doi: 10.1007/BF02868786. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Cephalosporin-sensitive penicillin-binding proteins of Staphylococcus aureus and Bacillus subtilis active in the conversion of [14C]penicillin G to [14C]phenylacetylglycine. J Biol Chem. 1979 Dec 10;254(23):12056–12061. [PubMed] [Google Scholar]
- Wilkinson B. J., Deans J. A., Ellar D. J. Biochemical evidence for the reversed polarity of the outer membrane of the bacterial forespore. Biochem J. 1975 Dec;152(3):561–569. doi: 10.1042/bj1520561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M., Mandelstam J. Early events during bacterial endospore formation. Adv Microb Physiol. 1979;20:103-62, 321-3. doi: 10.1016/s0065-2911(08)60207-6. [DOI] [PubMed] [Google Scholar]