Abstract
Ixolaris is a two-Kunitz tick salivary gland tissue factor pathway inhibitor (TFPI). In contrast to human TFPI, Ixolaris specifically binds to factor Xa (FXa) heparin-binding exosite (HBE). In addition, Ixolaris interacts with zymogen FX. In the present work we characterized the interaction of Ixolaris with human FX quantitatively, and identified a precursor state of the heparin-binding exosite (proexosite, HBPE) as the Ixolaris-binding site on the zymogen. Gel-filtration chromatography demonstrated 1:1 complex formation between fluorescein-labeled Ixolaris and FX. Isothermal titration calorimetry confirmed that the binding of Ixolaris to FX occurs at stoichiometric concentrations in a reaction which is characteristically exothermic, with a favorable enthalpy (ΔH) of −10.78 kcal/mol. ELISA and plasmon resonance experiments also indicate that Ixolaris binds to plasma FX and FXa, or to recombinant Gla domain-containing FX/FXa with comparable affinities (∼1 nM). Using a series of mutants on the HBPE, we identified the most important amino acids involved in zymogen/Ixolaris interaction—Arg-93 >>> Arg-165 ≥ Lys-169 > Lys-236 > Arg-125—which was identical to that observed for FXa/Ixolaris interaction. Remarkably, Ixolaris strongly inhibited FX activation by factor IXa in the presence but not in the absence of factor VIIIa, suggesting a specific interference in the cofactor activity. Further, solid phase assays demonstrated that Ixolaris inhibits FX interaction with immobilized FVIIIa. Altogether, Ixolaris is the first inhibitor characterized to date that specifically binds to FX HBPE. Ixolaris may be a useful tool to study the physiological role of the FX HBPE and to evaluate this domain as a target for anticoagulant drugs.
Keywords: tissue factor, heparin-binding exosite, proexosite, intrinsic tenase, prothrombinase
Coagulation factor X (FX) is a vitamin K-dependent zymogen that is activated into FXa by cleavage at a single Arg-15–Ile-16 peptide bond in the chymotrypsin numbering system (Bode et al. 1989). During the amplification phase, this reaction is mainly performed by the enzyme factor IXa (FIXa), which assembles with its protein cofactor, factor VIIIa (FVIIIa), on membrane surfaces in the presence of Ca2+ to form the intrinsic tenase complex (Mertens et al. 1999; Monroe et al. 2002). It is generally observed that phospholipids decrease the K m for FX while FVIIIa increases the K cat, resulting in an ∼105-fold increase in the catalytic efficiency of the intrinsic tenase complex compared to FIXa alone (Van Dieijen et al. 1981).
A striking feature in the clotting cascade is the great homology among the enzymes, most of them serine proteases, which display a trypsin-like catalytic domain (Rose and Di Cera 2002). Although sharing similarities in the catalytic as well as in other specific domains, coagulation proteinases act on their protein substrates with narrow and distinctive specificity (Bode et al. 1997). In particular, extended binding sites (exosites) play a crucial role in substrate, cofactor, or inhibitor recognition within the coagulation cascade (Krishnaswamy 2005). This is the molecular basis for the defined protein substrate specificity of coagulation enzyme complexes.
A number of studies indicate that certain surface-exposed residues on FXa are important mediators of the prothrombinase complex assembly, thus mediating the enzyme interaction with its protein cofactor, factor Va, and/or with its macromolecular substrate, prothrombin (Chattopadhyay et al. 1992; Betz and Krishnaswamy 1998; Rezaie 2000b; Yegneswaran et al. 2003). In addition, previous site-directed mutagenesis studies have indicated that the basic residues Arg-93, Lys-96, Arg-125, Arg-165, Lys-169, Lys-236, and Arg-240 constitute a region in the heavy chain of FXa, named heparin-binding exosite (HBE) (Rezaie 2000b). HBE can effectively bind heparin, thus mediating the enzyme inhibition by the antithrombin–heparin complex (Rezaie 2000a,b). On the other hand, FX exhibits a weak affinity for heparin, indicating that a precursor state of the HBE, namely heparin-binding proexosite (HBPE), is not exposed or is in a distinct conformation in the zymogen (Nogami et al. 2004).
It is important to recognize that FXa HBE is structurally similar to the well-studied heparin-binding site of thrombin (Padmanabhan et al. 1993) also known as thrombin-anion binding exosite 2 (TABE2) (Sheehan and Sadler 1994). Another positively charged region, thrombin-anion binding exosite 1 (TABE1), is partially exposed on prothrombin, as a precursor state known as proexosite 1. Proexosite 1 exposure is critically dependent on prothrombin activation (Liu et al. 1991; Wu et al. 1994) with an ∼100-fold increase in affinity for specific ligands (Anderson et al. 2000a,b; Monteiro et al. 2001; Monteiro and Zingali 2002). On the other hand, crystallographic studies indicate that TABE2 is not accessible to heparin on prothrombin (Martin et al. 1997).
Exogenous ligands of coagulation factor exosites have also been identified from the salivary gland of blood-sucking arthropods (Fuentes-Prior et al. 1997; Bergum et al. 2001; Ribeiro and Francischetti 2003). Ixolaris, a tick salivary 140-amino acid protein containing 10 cysteines and two Kunitz-like domains, binds to FXa or FX as scaffolds and inhibits TF–FVIIa complex (Francischetti et al. 2002). In contrast to tissue factor pathway inhibitor (TFPI) (Broze Jr. 1995), however, Ixolaris does not bind to the active site cleft of FXa. Instead, complex formation is mediated by the FXa HBE (Monteiro et al. 2005). In addition, Ixolaris interacts with zymogen FX through an unidentified region. Accordingly, we have further studied the mechanism of Ixolaris interaction with FX. Our results show that Ixolaris specifically targets a precursor state of HBPE of the zymogen FX. Our data also provide strong evidence that HBPE of FX mediates the interaction of the zymogen with FVIIIa, accounting for the productive recognition of the substrate by the intrinsic tenase complex.
Results
Complex formation between Ixolaris and FX
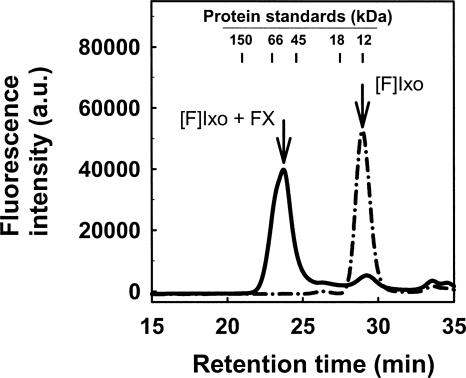
Binding of Ixolaris to human FX was examined first by Superose 12 gel filtration, using a fluorescein-labeled derivative of Ixolaris ([F]-Ixolaris) to monitor elution (Fig. 1, dotted line). In a typical experiment, the retention time of [F]-Ixolaris was ∼29 min, which is consistent with the previously determined molecular mass of the protein (15.5 kDa) (Francischetti et al. 2002). When Ixolaris and FX were incubated at stoichiometric proportions and loaded into the column, a peak of ∼24.5 min was obtained (Fig. 1, solid line). Calibration curves performed with proteins of known molecular weights indicate that these results were consistent with formation of a 1:1 complex of ∼75 kDa between [F]-Ixolaris (15.5 kDa) and FX (59 kDa).
Figure 1.
Complex formation between Ixolaris and FX investigated by gel-filtration chromatography. Gel-filtration chromatography on Superose 12 was performed at a flow rate of 0.5 mL/min. Elution of [F]-Ixolaris (2 μM) was monitored by the elution time of the fluorescein fluorescence in the absence (dash-dotted line) or in the presence (solid line) of FX (2 μM). Arrows indicate the retention time of [F]-Ixolaris ([F]Ixo) and the complex [F]-Ixolaris–FX. a.u., Arbitrary units.
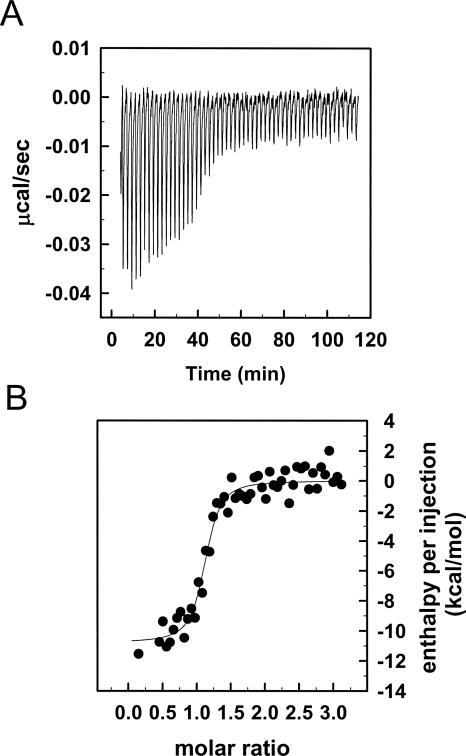
Binding of Ixolaris to FX was measured by isothermal titration calorimetry (ITC) with the results shown in Figure 2. Analysis of the results according to a single set of identical binding sites gave a dissociation constant (K D) of 11.7 ± 4.5 nM for Ixolaris binding to FX. Binding was exothermic, with a favorable enthalpy (ΔH) of −10.78 kcal/mol. These data allowed the calculation of the free-energy (ΔG = −11.0 kcal/mol) and entropy (ΔS = 0.734 cal/mol K) for binding of the zymogen to the inhibitor. In agreement with the results obtained by gel-filtration chromatography, the stoichiometry of the binding (n = 1.11 ± 0.02) indicates that one FX molecule binds to one Ixolaris molecule.
Figure 2.
Solution binding of Ixolaris to human FX as measured by isothermal titration calorimetry. (A) Baseline adjusted heats per injection of FX (14.1 μM) into Ixolaris (1.0 μM). (B) Molar enthalpies per injection for FX interaction with Ixolaris. Filled circles, measured enthalpies; solid line, fit of experimental data to a single site-binding model. Thermodynamic parameters: ΔH = −10.78 ± 3.44 kcal/mol, TΔS = 2.22 kcal/mol, K(assoc) = 8.57 ± 2.58 × 107.
To investigate binding kinetics of Ixolaris–FX/FXa interactions, surface plasmon resonance (SPR) experiments were performed. Typical sensorgrams obtained for Ixolaris interaction with FX and FXa, respectively, are shown in Figure 3A,B. In both cases, the best fit was attained using a two-state reaction model (Table 1), suggesting that conformational change occurs after inhibitor binding to the coagulation factors. This is consistent with the observation that Ixolaris is a slow-tight ligand of FX (Francischetti et al. 2002). Using this model, K D of 0.67 nM for FX and K D of 0.25 nM for FXa were calculated (Table 1).
Figure 3.
Ixolaris binds to factor X and factor Xa. Typical sensorgrams of Ixolaris interaction with factor X (A) and factor Xa (B). Different concentrations of FX (in nM: a, 250; b, 125; c, 62.5; d, 31.75; and e, 15) or FXa (in nM: a, 62.5; b, 31.75; c, 15; d, 7.5; and e, 3.75) were injected over immobilized Ixolaris for 180 s. Dissociation of Ixolaris–FX/Xa complex was monitored for 2000 s, and a global two-state binding model was used to calculate kinetic parameters. Representative sensorgrams are shown. RU, resonance units.
Table 1.
Kinetics of Ixolaris interaction with factor X and factor Xa estimated by SPR
Ixolaris binds to FX via basic residues that comprise the FXa heparin-binding exosite
We have previously demonstrated that Ixolaris binds to FXa through residues that constitute the HBE of the enzyme (Monteiro et al. 2005). In an attempt to evaluate whether residues found in FXa HBE mediate Ixolaris interaction with the zymogen, an ELISA was optimized as described in Materials and Methods. Accordingly, we measured the binding of recombinant wild-type or plasma-derived forms of FXa and FX (0–100 nM) to immobilized Ixolaris (Fig. 4). Data were analyzed according to the Langmuir binding isotherm, yielding similar dissociation constants for recombinant or plasma proteins with a calculated K D of 1.8 nM and K D of 0.81 nM for interaction of Ixolaris with FX or FXa, respectively (Table 2). Controls performed with wild-type FX preincubated with Ixolaris showed no binding, demonstrating the specificity of the assay (data not shown).
Figure 4.
Ixolaris interaction with FXa or FX derivatives. An ELISA-based assay using 96-well microtiter plates coated with Ixolaris (10 ng/mL) was used to measure the affinity of the inhibitor for interaction with either wild type or mutants of (A) FX or (B) FXa, as described in Materials and Methods. The symbols are: plasma (○), wild type (●), R93A (□), R125A (■), R165A (△), K169A (▲), and K236A (▽). In B, the data points for plasma FXa (○) are not seen because the results were identical to wild-type FXa (●) and the symbols were superimposed.
Table 2.
Complex formation between Ixolaris, plasma FX/FXa, recombinant wild-type FX/FXa, and mutants of FX/HBPE or FXa/HBE
Next, we evaluated the affinity of FXa HBE or FX HBPE mutants containing Gla domain, for interaction with Ixolaris. As previously demonstrated, all mutations interfered with complex formation between Ixolaris and FXa (Monteiro et al. 2005), with calculated affinities ranging from 8.72 to 29.60 nM. Mutations caused similar effects in the Ixolaris/FXa interaction and, remarkably, the order of importance for complex formation was identical for both the zymogen and the enzyme forms. For instance, it was observed that mutation on residue Arg-93 completely abolished the interaction of the FXa or FX mutants with Ixolaris. Table 2 summarizes our findings.
Effect of Ixolaris on FX activation
In order to evaluate whether complex formation between Ixolaris and FX could interfere with zymogen activation, we tested the effect of Ixolaris in the intrinsic tenase complex. Figure 5A shows that premixing of Ixolaris with FX (150 nM) produced a concentration-dependent inhibition of FX activation by the assembled intrinsic tenase complex, and maximum inhibition was observed at stoichiometric concentrations of zymogen and inhibitor (∼150 nM). On the other hand, Figure 5B shows that no effect was observed when FIXa activation assays were performed in the absence of FVIIIa. This finding strongly suggests that the inhibitor interferes with FX–cofactor interactions.
Figure 5.
Effect of Ixolaris on FX activation by FIXa. (A) Time course of FX (150 nM) activation by FIXa (0.1 nM), FVIIIa (10 U/mL), and 10 μM PCPS was assayed in a discontinuous chromogenic assay using the FXa substrate S-2222 (200 μM), as described in Materials and Methods. Zymogen activation reactions were carried out in the presence of Ixolaris as follows: 0 nM (●), 50 nM (■), 75 nM (▲), 125 nM (▼), 150 nM (♦), and 200 nM (○). (B) Effect of Ixolaris on FX (150 nM) activation by FIXa (0.1 nM), FVIIIa (10 U/mL), and 10 μM PCPS (■) or FX (150 nM) activation by FIXa (10 nM) and 10 μM PCPS (●) was assayed as described above at the indicated concentrations of Ixolaris. For each condition, FX activation in the absence of Ixolaris was taken as 100%.
Ixolaris prevents FX interaction with FVIIIa
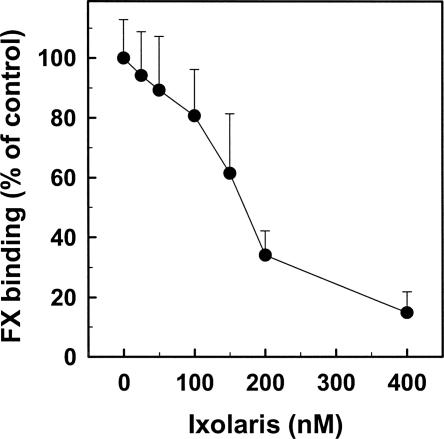
To test whether Ixolaris interferes with FX–FVIIIa interaction, a solid phase assay was carried out as described in the Materials and Methods section. Figure 6 shows that Ixolaris dose-dependently inhibits FX binding to immobilized FVIIIa, a finding that is consistent with a tight interaction of the zymogen with the inhibitor (see Discussion).
Figure 6.
Inhibition of FX binding to FVIIIa by Ixolaris. Mixtures of 200 nM FX and varying amounts of Ixolaris were reacted with FVIIIa (50 U/mL) that had been immobilized onto microtiter wells. Bound FX was detected after the addition of 30 nM RVV-X and chromogenic substrate as described in Materials and Methods. FX binding to FVIIIa in the absence of Ixolaris was taken as 100%.
Discussion
In a previous report we showed that Ixolaris, a two-Kunitz protein from tick salivary gland, specifically recognizes FXa HBE, producing a highly stable noncovalent complex (Monteiro et al. 2005). As a result, prothrombin recognition by the enzyme is severely impaired, leading to a significant inhibition of zymogen activation. Previous observations also identified a stable complex between Ixolaris and zymogen FX either in a purified system (Francischetti et al. 2002) or in plasma (Nazareth et al. 2006).
Herein we have shown that Ixolaris interacts with high affinity to FX or FXa (K D ∼ 1 nM) as estimated by ELISA. Similar results were obtained with an independent estimate of K D for FX and FXa (K D ∼ 0.5 nM) using SPR and a different set of experiments and equations. In addition, the K D observed by titration calorimetry (K D ∼ 10 nM) was in reasonable agreement with the affinity obtained above, considering the limitations on the amount of the protein components needed to see observable heat. Notably, Ixolaris interacts with a precursor state of FXa HBE, or FX HBPE. This conclusion is supported using a series of Gla domain-containing FX derivatives mutated on HBPE. The order of importance for Ixolaris/zymogen recognition was Arg-93 >>> Arg-165 ≥ Lys-169 > Lys-236 > Arg-125. The relative contribution of basic residues in the FX/Ixolaris interaction was essentially identical to that reported for FXa/Ixolaris complex formation (Monteiro et al. 2005). These findings suggest that the interaction of Ixolaris with FXa or FX is mediated by similar residues in the FXa HBE and FX HBPE, which appears to be equally accessible to the inhibitor in the zymogen or in the enzyme. Further, affinity of Ixolaris for FX or FXa is very similar, and this finding distinguishes the interaction between heparin and Ixolaris with the FX HBPE. In fact, while heparin binds to FXa, it does so with lower affinity to FX, suggesting that HBPE is not readily exposed to heparin or is in a distinct conformation in the zymogen (Rezaie 2000b; Nogami et al. 2004).
The finding that Ixolaris interacts with the HBPE has mechanistic implications for its anticoagulant activity. Several experiments indicate that FX interacts with the A1 domain of FVIIIa, the primary cofactor of the intrinsic Xnase complex (Lapan and Fay 1997, 1998; Nogami et al. 2004). It has been reported that the sequence 337–372 of the FVIIIa A1 subunit contains interactive sites for both FX and FXa (Nogami et al. 2004). Further, heparin prevents cross-linking of the 337–372 peptide with FXa while the R240A FXa HBE mutant is defective in recognizing the Lys-36 cleavage site on the A1 domain of FVIIIa. In other words, HBE seems to mediate cofactor interaction with FXa which displays an ∼20-fold higher affinity for FVIIIa A1 domain than FX (Nogami et al. 2004). In this context, we observed that complex formation between Ixolaris and FX attenuates zymogen activation by the assembled tenase complex but not in the absence of FVIIIa (Fig. 5). It is plausible that the FX binding sites for FVIIIa are recognized by Ixolaris, a contention supported by the results presented in Figure 6. However, it also appears that secondary sites, not readily recognized by Ixolaris, mediate the binding of FX to FVIIIa. Accordingly, FX (200 nM), when saturated with Ixolaris (200 nM), displays ∼25% residual binding to the cofactor (Fig. 5). It is concluded that Ixolaris affects FX binding to FVIIIa on the one hand (this work), and FXa interaction with prothrombin on the other (Monteiro et al. 2005). Exosite-targeted inhibition appears to be an effective strategy evolved by blood-sucking arthropods to effectively block the coagulation cascade, which in turn assists successful blood-feeding (Ribeiro and Francischetti 2003).
Concerning the functional role of HBPE of FX in the intrinsic tenase complex, experiments have demonstrated that R240A FX mutant and wild-type FX are equally activated by FIXa, FVIIIa, PC/PS (l-α-phosphatidylcholine/l-α-phosphatidylserine), and Ca2+ (Nogami et al. 2004). This observation suggests that R240A has a minor functional contribution in zymogen–cofactor interaction. Our experiments also indicate that single-point mutations on HBPE residues of FX only slightly affected Ixolaris inhibitory activity toward the extrinsic or intrinsic tenase complexes (data not show). Since FX HBPE mutants displayed clear reduction in the affinity for Ixolaris, as demonstrated in protein–protein interaction assays (Fig. 4), it is possible that single mutations are not sufficient to cause a significant decrease in the inhibitor–zymogen interaction in the assembled intrinsic tenase. This is more likely due to the dominant role of PCPS vesicles in reducing the K m of the reactions (Van Dieijen et al. 1981) and thus overcoming the catalytic defects of the activation complexes toward the FX mutant zymogens in the presence of Ixolaris.
The ability of Ixolaris to interact with FX in plasma is mechanistically relevant since it allows the inhibitor to circulate bound to the zymogen whose half-life is >30 h, which therefore contributes to the long-lasting antithrombotic effect of the inhibitor (Nazareth et al. 2006). In fact, we have demonstrated that Ixolaris decreases thrombus formation in an animal model even after 24 h of subcutaneous administration (Nazareth et al. 2006). The fact that Ixolaris barely affects the aPTT at effective antithrombotic concentrations (Nazareth et al. 2006) suggests that Ixolaris binding to FX may not significantly affect the intrinsic Xnase in vivo, but rather form an FX–Ixolaris complex that inhibits FVIIa/TF with high affinity even if only a fraction of the zymogen is bound to the inhibitor. These results support further consideration of Ixolaris as an effective and possibly safe antithrombotic/anticoagulant in different condition with abnormal expression of tissue factor (Lupu et al. 2005; Francischetti et al. 2007; White et al. 2007).
Materials and Methods
Proteins and chemicals
Recombinant Ixolaris was produced in High Five cells (Invitrogen), purified, and quantified as previously described (Monteiro et al. 2005). Full-length wild-type and the Ala-substitution mutants of FX or FXa derivatives (chymotrypsin numbering)—Arg-93 → Ala (R93A), Arg-125 → Ala (R125A), Arg-165 → Ala (R165A), Lys-169 → Ala (K169A), and Lys-236 → Ala (K236A)—were expressed in human embryonic kidney (HEK-293) cells as described previously (Chen et al. 2003). All derivatives were purified to homogeneity by a combination of immunoaffinity followed by the Mono Q ion exchange chromatography as described (Chen et al. 2004). The purity of all recombinant proteins was confirmed by SDS-PAGE under nonreducing conditions. Human FIXa, FX, and FXa were purchased from Haematologic Technologies. Human FVIII (Advate) was from Baxter Healthcare Corporation. FVIII was activated with human thrombin as previously described (Astermark et al. 1992). S-2222 was from Chromogenix; l-α-phosphatidylcholine (PC) and l-α-phosphatidylserine (PS) were from Sigma Chemical Co. Phospholipid vesicles (PC/PS) composed of 75% PC, 25% PS (w/w) were prepared by sonication as described (Monteiro et al. 2005). Briefly, phospholipids in chloroform were dried with a stream of N2, resuspended in 50 mM Tris-HCl, 150 mM NaCl, pH 7.5, and sonicated for 2 min. Solution was further adjusted to 500 μM, final concentration.
Gel filtration
Gel-filtration chromatography was performed using a Superose 12 HR column (Pharmacia) attached to a Shimadzu high-pressure liquid chromatography system. The column was equilibrated with 50 mM Tris-HCl, 150 mm NaCl, pH 7.5 at a flow rate of 0.5 mL/min. [F]-Ixolaris was prepared by incubating the protein with fluorescein succinimidyl ester (PanVera), according to the manufacturer's instructions. Samples containing [F]-Ixolaris or [F]-Ixolaris/FX were preincubated at room temperature for 20 min prior to 20-μL injections. Elution was followed using two detectors: one for absorbance at 280 nm and another for fluorescein fluorescence (excitation at 490 nm, emission at 510 nm). Calibration of the gel filtration column was performed with the following proteins: vicilin (150 kDa), bovine serum albumin (66 kDa), ovalbumin (45 kDa), beta lactoglobulin (18 kDa), and cytochrome C (12 kDa).
Isothermal titration calorimetry
Calirometric assays for measuring FX binding to Ixolaris were performed using a Microcal VP-ITC microcalorimeter at 30°C. Titration experiments were performed by making successive injections of 5 μL each of 14.1 μM FX into the 1.34 mL sample cell containing 1 μM Ixolaris until near-saturation was achieved. Prior to the run, the proteins were dialyzed against 20 mM Tris-HCl, 0.15 M NaCl, pH 7.4, for binding experiments. The calorimetric enthalpy (ΔH cal) for each injection was calculated after correction for the heat of FX dilution obtained in control experiments performed by titrating FX into buffer.
The binding isotherms were fitted according to a model for a single set of identical binding sites by nonlinear squares analysis using Microcal Origin software. The enthalpy change (ΔH) and stoichiometry (n) were determined according to Equation 1:
where Q is the total heat content of the solution contained in the cell volume (Vo), at fractional saturation θ, ΔH is the molar heat of ligand binding, n is the number of sites, and Mt is the bulk concentration of macromolecule in Vo. The binding constant, K a, is described as:
where [X] is the free concentration of ligand.
The free-energy (ΔG) and entropy term (−TΔS) of association were calculated according to:
Enzyme-linked immunosorbent assays (ELISA)
To evaluate the interaction of Ixolaris with FX, 96-well flat microtiter plates were coated with Ixolaris (10 ng/mL) in ELISA buffer (TBS containing 1 mM CaCl2) overnight at 4°C. After the plates were washed three times with the same TBS buffer containing 0.05% Tween 20, they were incubated with 1% BSA in the Ca2+-containing TBS buffer for 2 h at room temperature. After the plates were washed as described above, they were incubated with each FX or FXa protein (0–100 nM) diluted in TBS/Ca2+ containing 0.1% BSA for 1 h. The plates were rinsed and incubated with goat polyclonal antibody against FX (1 μg/mL) for 1 h. The plates were then washed and incubated with rabbit anti-goat IgG (1:1000) (KPL) for 1 h. After washing, the plates were incubated with 2,2′-azino-di(3-ethylbenzthiazoline-6-sulfonate) (ABTS; KPL). Colorimetric analysis was performed by measuring the absorbance values at 405 nm. The (apparent) Kd values for Ixolaris/FX interaction were calculated by nonlinear regression analysis of the binding data according to the Langmuir isotherm equation. All treatments were performed in duplicate and repeated at least three times.
Surface plasmon resonance (SPR) analysis
All SPR experiments were carried out in a T100 instrument (Biacore Inc.) following the manufacturer's instructions. This instrument features an integrated degasser, allowing problem-free kinetic measurements at temperatures up to 45°C, as well as a temperature-controlled flow cell and sample compartment. The Biacore T100 evaluation software was utilized for kinetic and thermodynamic evaluation. Sensor CM5, amine coupling reagents, and buffers were also purchased from Biacore Inc. HBS-P (10 mM HEPES, pH 7.4, 150 mM NaCl, and 0.005% [v/v] P20 surfactant) was used as the running buffer for all SPR experiments. All SPR experiments were carried out three times.
Immobilization and kinetic analysis
Ixolaris (30 μg/mL) in acetate buffer pH 4.0 was immobilized over a CM5 sensor via amine coupling, resulting in a final immobilization of 566.9 RU. Blank flow cells were used to subtract the buffer effect on sensorgrams. Kinetic experiments were carried out with a contact time of 180 s at a flow rate of 30 μL/min at 25°C. Ixolaris–FX/FXa complex dissociation was monitored for 2000 s, and the sensor surface was regenerated by a pulse of 60 s of 20 mM HCl at 30 μL/min. Sensorgrams were fitted using the two-state reaction (conformational change) interaction model as follows:
 |
Model parameters are: Ka1, association rate constant for analyte binding; kd1, dissociation rate constant for analyte from the complex; ka2, forward rate constant for the conformational change; kd2, reverse rate constant for the conformational change.
FX activation by FIXa
Activation of human FX to FXa by FIXa was performed in 50 mm Tris-HCl, 150 mm NaCl, 10 mm CaCl2, 0.1% PEG 6000, pH 7.5, using a discontinuous assay. FX (150 nm, final concentration) was incubated with different Ixolaris concentrations for 15 min at 37°C. Reaction was started by addition of a mixture containing FIXa (0.1 nM, final concentration), FVIIIa (10 U/mL), and 10 μM PCPS. Aliquots of 25 μL were removed every 30 s into microplate wells containing 25 μL of 50 mm Tris-HCl, 150 mm NaCl, 50 mm EDTA, 0.1% PEG 6000, pH 7.5. After addition of 50 μL of 200 μM S-2222, absorbance at 405 nm was recorded, at 37°C, for 10 min at 6-s intervals using a Thermomax Microplate Reader (Molecular Devices) equipped with a microplate mixer and heating system as described (Francischetti et al. 1999). Velocities (mOD/min) obtained in the first minutes of reaction were used to calculate the amount of FXa formed. Experiments in the absence of FVIIIa were performed as follows: FX (150 nm, final concentration) was incubated with different Ixolaris concentrations for 15 min at 37°C. Reaction was started by addition of a mixture containing FIXa (10 nM, final concentration) and 10 μM PCPS. Aliquots of 25 μL were removed after 30 min into microplate wells containing 25 μL of 50 mm Tris-HCl, 150 mm NaCl, 50 mm EDTA, 0.1% PEG 6000, pH 7.5. The amount of FXa formed was determined as described above.
Factor VIIIa solid phase binding assay
The direct binding assay of FX to immobilized FVIIIa was performed using a previously reported method (Nogami et al. 2004) with modifications. All reactions were run at 22°C. FVIIIa (50 U/mL) was bound to the microtiter wells in 15 mM Na2CO3, 35 mM NaHCO3, pH 9.6, overnight at 4°C. After washing the wells with washing buffer (20 mM HEPES, pH 7.2, 15 mM NaCl, 5 mM CaCl2, and 0.01% Tween 20), wells were blocked in with 5% BSA for 2 h. After washing three times, mixtures of 200 nM FX and varying amounts of Ixolaris (in washing buffer containing 1% BSA) were added to the wells and incubated for 60 min. After several washings, 30 nM FX activating enzyme from Russell's viper venom (RVV-X) was added and incubated for 30 min. This was followed by the addition of the chromogenic substrate, S-2222 (0.1 mM, final concentration), and the plate was read at 405 nm for 60 min using a Thermomax Microplate reader (Francischetti et al. 1999). Control experiments showed that the RVV-X did not hydrolyze the chromogenic substrate. Specific binding was obtained by subtracting nonspecific binding of FX.
Acknowledgments
We thank Drs. José Ribeiro, Robert Gwadz, Thomas Wellems, and Kathryn Zoon (NIAID/NIH) for encouragement and support, and Van My Pham for technical assistance. For financial support we acknowledge the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), the National Heart, Lung, and Blood Institute of the National Institutes of Health (grant HL 68571 to A.R.R.), and the Intramural Research Program of the National Institutes of Allergy and Infectious Diseases/NIH.
Footnotes
Reprint requests to: Ivo M.B. Francischetti, Laboratory of Malaria and Vector Research, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, 12735 Twinbrook Parkway, Twinbrook III Building, Room 2E-28, Bethesda, MD 20892-8132, USA; e-mail: ifrancischetti@niaid.nih.gov; fax: (301) 480-2571.
Abbreviations: [F]-Ixolaris, Ixolaris labeled at the N-terminal by fluorescein; FX, factor X; FXa, factor Xa; HBE, heparin-binding exosite; ITC, isothermal titration calorimetry; PC, l-α-phosphatidylcholine; PEG, polyethylene glycol; PS, l-α-phosphatidylserine; S-2222, Bz-Ile-Glu(γ-OR)-Gly-Arg-pNA·HCl; TABE, thrombin-anion binding exosite; TFPI, tissue factor pathway inhibitor; SPR, surface plasmon resonance.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.073016308.
References
- Anderson, P.J., Nesset, A., Dharmawardana, K.R., Bock, P.E. Characterization of proexosite I on prothrombin. J. Biol. Chem. 2000a;275:16428–16434. doi: 10.1074/jbc.M001254200. [DOI] [PubMed] [Google Scholar]
- Anderson, P.J., Nesset, A., Dharmawardana, K.R., Bock, P.E. Role of proexosite I in factor Va-dependent substrate interactions of prothrombin activation. J. Biol. Chem. 2000b;275:16435–16442. doi: 10.1074/jbc.M001255200. [DOI] [PubMed] [Google Scholar]
- Astermark, J., Hogg, P.J., Bjork, I., Stenflo, J. Effects of γ-carboxyglutamic acid and epidermal growth factor-like modules of factor IX on factor X activation. J. Biol. Chem. 1992;267:3249–3256. [PubMed] [Google Scholar]
- Bergum, P.W., Cruikshank, A., Maki, S.L., Kelly, C.R., Ruf, W., Vlasuk, G.P. Role of zymogen and activated factor X as scaffolds for the inhibition of the blood coagulation factor VIIa-tissue factor complex by recombinant nematode anticoagulant protein c2. J. Biol. Chem. 2001;276:10063–10071. doi: 10.1074/jbc.M009116200. [DOI] [PubMed] [Google Scholar]
- Betz, A., Krishnaswamy, S. Regions remote from the site of cleavage determine macromolecular substrate recognition by the prothrombinase complex. J. Biol. Chem. 1998;273:10709–10718. doi: 10.1074/jbc.273.17.10709. [DOI] [PubMed] [Google Scholar]
- Bode, W., Mayr, I., Baumann, U., Huber, R., Stone, S.R., Hofsteenge, J. The refined 1.9 Å crystal structure of human α-thrombin: Interaction with D-Phe-Pro-Arg chlorometheylketone and significance of the Tyr-Pro-Pro-Trp insertion segment. EMBO J. 1989;8:3467–3475. doi: 10.1002/j.1460-2075.1989.tb08511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode, W., Brandstetter, H., Mather, T., Stubbs, M.T. Comparative analysis of haemostatic proteinases: Structural aspects of thrombin, factor Xa, factor IXa and protein C. Thromb. Haemost. 1997;78:501–511. [PubMed] [Google Scholar]
- Broze G.J., Jr Tissue factor pathway inhibitor and the revised theory of coagulation. Annu. Rev. Med. 1995;46:103–112. doi: 10.1146/annurev.med.46.1.103. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay, A., James, H.L., Fair, D.S. Molecular recognition sites on factor Xa which participate in the prothrombinase complex. J. Biol. Chem. 1992;267:12323–12329. [PubMed] [Google Scholar]
- Chen, L., Yang, L., Rezaie, A.R. Proexosite-1 on prothrombin is a factor Va-dependent recognition site for the prothrombinase complex. J. Biol. Chem. 2003;278:27564–27569. doi: 10.1074/jbc.M302707200. [DOI] [PubMed] [Google Scholar]
- Chen, L., Manithody, C., Yang, L., Rezaie, A.R. Zymogenic and enzymatic properties of the 70–80 loop mutants of factor X/Xa. Protein Sci. 2004;13:431–442. doi: 10.1110/ps.03406904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti, I.M., Valenzuela, J.G., Ribeiro, J.M. Anophelin: Kinetics and mechanism of thrombin inhibition. Biochemistry. 1999;38:16678–16685. doi: 10.1021/bi991231p. [DOI] [PubMed] [Google Scholar]
- Francischetti, I.M., Valenzuela, J.G., Andersen, J.F., Mather, T.N., Ribeiro, J.M. Ixolaris, a novel recombinant tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick, Ixodes scapularis: Identification of factor X and factor Xa as scaffolds for the inhibition of factor VIIa/tissue factor complex. Blood. 2002;99:3602–3612. doi: 10.1182/blood-2001-12-0237. [DOI] [PubMed] [Google Scholar]
- Francischetti, I.M., Seydel, K.B., Monteiro, R.Q., Whitten, R.O., Erexson, C.R., Noronha, A.L., Ostera, G.R., Kamiza, S.B., Molyneux, M.E., Ward, J.M., et al. Plasmodium falciparum-infected erythrocytes induce tissue factor expression in endothelial cells and support the assembly of multimolecular coagulation complexes. J. Thromb. Haemost. 2007;5:155–165. doi: 10.1111/j.1538-7836.2006.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Prior, P., Noeske-Jungblut, C., Donner, P., Schleuning, W.D., Huber, R., Bode, W. Structure of the thrombin complex with triabin, a lipocalin-like exosite-binding inhibitor derived from a triatomine bug. Proc. Natl. Acad. Sci. 1997;94:11845–11850. doi: 10.1073/pnas.94.22.11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaswamy, S. Exosite-driven substrate specificity and function in coagulation. J. Thromb. Haemost. 2005;3:54–67. doi: 10.1111/j.1538-7836.2004.01021.x. [DOI] [PubMed] [Google Scholar]
- Lapan, K.A., Fay, P.J. Localization of a factor X interactive site in the A1 subunit of factor VIIIa. J. Biol. Chem. 1997;272:2082–2088. doi: 10.1074/jbc.272.4.2082. [DOI] [PubMed] [Google Scholar]
- Lapan, K.A., Fay, P.J. Interaction of the A1 subunit of factor VIIIa and the serine protease domain of factor X identified by zero-length cross-linking. Thromb. Haemost. 1998;80:418–422. [PubMed] [Google Scholar]
- Liu, L.W., Ye, J., Johnson, A.E., Esmon, C.T. Proteolytic formation of either of the two prothrombin activation intermediates results in formation of a hirugen-binding site. J. Biol. Chem. 1991;266:23632–23636. [PubMed] [Google Scholar]
- Lupu, C., Westmuckett, A.D., Peer, G., Ivanciu, L., Zhu, H., Taylor F.B., Jr, Lupu, F. Tissue factor-dependent coagulation is preferentially up-regulated within arterial branching areas in a baboon model of Escherichia coli sepsis. Am. J. Pathol. 2005;167:1161–1172. doi: 10.1016/S0002-9440(10)61204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, P.D., Malkowski, M.G., Box, J., Esmon, C.T., Edwards, B.F. New insights into the regulation of the blood clotting cascade derived from the X-ray crystal structure of bovine meizothrombin des F1 in complex with PPACK. Structure. 1997;15:1681–1693. doi: 10.1016/s0969-2126(97)00314-6. [DOI] [PubMed] [Google Scholar]
- Mertens, K., Celie, P.H., Kolkman, J.A., Lenting, P.J. Factor VIII-factor IX interactions: Molecular sites involved in enzyme-cofactor complex assembly. Thromb. Haemost. 1999;82:209–217. [PubMed] [Google Scholar]
- Monroe, D.M., Hoffman, M., Roberts, H.R. Platelets and thrombin generation. Arterioscler. Thromb. Vasc. Biol. 2002;22:1381–1389. doi: 10.1161/01.atv.0000031340.68494.34. [DOI] [PubMed] [Google Scholar]
- Monteiro, R.Q., Zingali, R.B. Bothrojaracin, a proexosite I ligand, inhibits factor Va-accelerated prothrombin activation. Thromb. Haemost. 2002;87:288–293. [PubMed] [Google Scholar]
- Monteiro, R.Q., Bock, P.E., Bianconi, M.L., Zingali, R.B. Characterization of bothrojaracin interaction with human prothrombin. Protein Sci. 2001;10:1897–1904. doi: 10.1110/ps.09001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro, R.Q., Rezaie, A.R., Ribeiro, J.M., Francischetti, I.M. Ixolaris: A factor Xa heparin-binding exosite inhibitor. Biochem. J. 2005;387:871–877. doi: 10.1042/BJ20041738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazareth, R.A., Tomaz, L.S., Ortiz-Costa, S., Atella, G.C., Ribeiro, J.M., Francischetti, I.M., Monteiro, R.Q. Antithrombotic properties of Ixolaris, a potent inhibitor of the extrinsic pathway of the coagulation cascade. Thromb. Haemost. 2006;96:7–13. doi: 10.1160/TH06-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogami, K., Freas, J., Manithody, C., Wakabayashi, H., Rezaie, A.R., Fay, P.J. Mechanisms of interactions of factor X and factor Xa with the acidic region in the factor VIII A1 domain. J. Biol. Chem. 2004;279:33104–33113. doi: 10.1074/jbc.M405537200. [DOI] [PubMed] [Google Scholar]
- Padmanabhan, K., Padmanabhan, K.P., Tulinsky, A., Park, C.H., Bode, W., Huber, R., Blankenship, D.T., Cardin, A.D., Kisiel, W. Structure of human des(1-45) factor Xa at 2.2 Å resolution. J. Mol. Biol. 1993;232:947–996. doi: 10.1006/jmbi.1993.1441. [DOI] [PubMed] [Google Scholar]
- Rezaie, A.R. Heparin-binding exosite of factor Xa. Trends Cardiovasc. Med. 2000a;10:333–338. doi: 10.1016/s1050-1738(01)00070-6. [DOI] [PubMed] [Google Scholar]
- Rezaie, A.R. Identification of basic residues in the heparin-binding exosite of factor Xa critical for heparin and factor Va binding. J. Biol. Chem. 2000b;275:3320–3327. doi: 10.1074/jbc.275.5.3320. [DOI] [PubMed] [Google Scholar]
- Ribeiro, J.M.C., Francischetti, I.M.B. Role of arthropod saliva in blood feeding: Sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- Rose, T., Di Cera, E. Substrate recognition drives the evolution of serine proteases. J. Biol. Chem. 2002;277:19243–19246. doi: 10.1074/jbc.C200132200. [DOI] [PubMed] [Google Scholar]
- Sheehan, J.P., Sadler, J.E. Molecular mapping of the heparin-binding exosite of thrombin. Proc. Natl. Acad. Sci. 1994;91:5518–5522. doi: 10.1073/pnas.91.12.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dieijen, G., Tans, G., Rosing, J., Hemker, H.C. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J. Biol. Chem. 1981;256:3433–3442. [PubMed] [Google Scholar]
- White, R.J., Meoli, D.F., Swarthout, R.F., Kallop, D.Y., Galaria, I.I., Harvey, J.L., Miller, C.M., Blaxall, B.C., Hall, C.M., Pierce, R.A., et al. Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L583–L590. doi: 10.1152/ajplung.00321.2006. [DOI] [PubMed] [Google Scholar]
- Wu, Q., Picard, V., Aiach, M., Sadler, J.E. Activation-induced exposure of the thrombin anion-binding exosite. J. Biol. Chem. 1994;269:3725–3730. [PubMed] [Google Scholar]
- Yegneswaran, S., Mesters, R.M., Griffin, J.H. Identification of distinct sequences in human blood coagulation factor Xa and prothrombin essential for substrate and cofactor recognition in the prothrombinase complex. J. Biol. Chem. 2003;278:33312–33318. doi: 10.1074/jbc.M305906200. [DOI] [PubMed] [Google Scholar]