Abstract
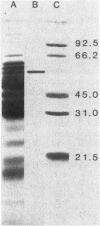
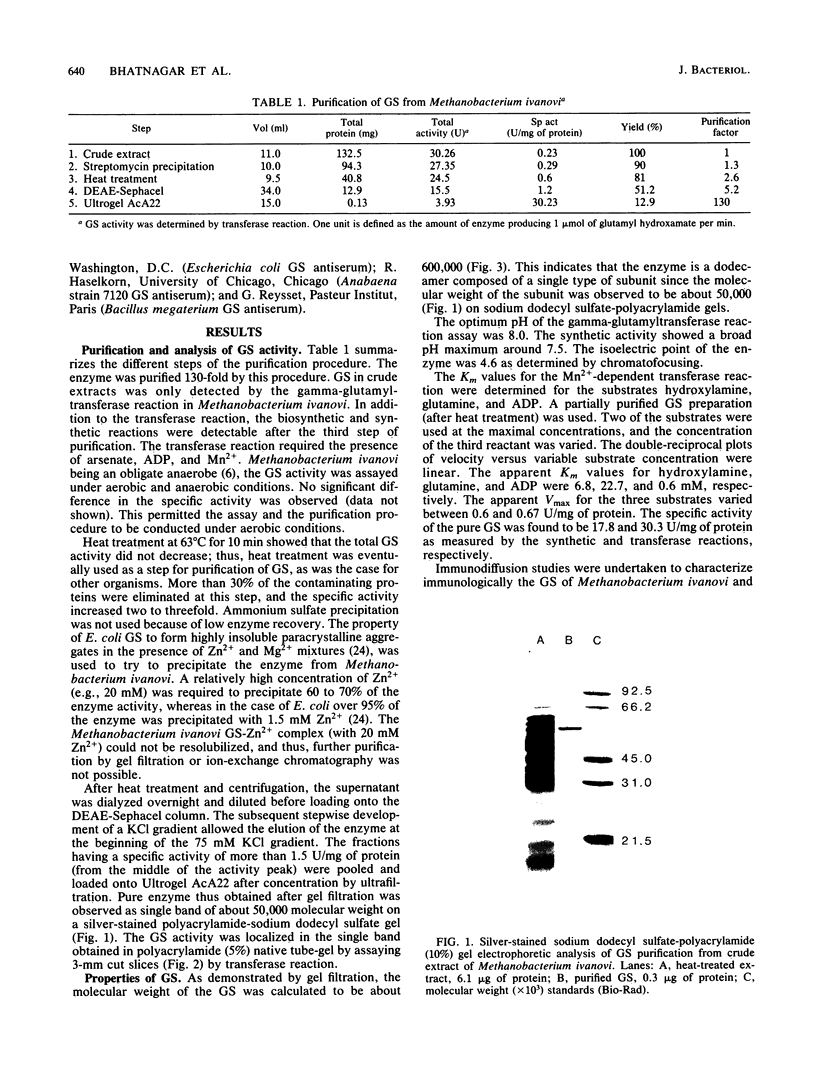
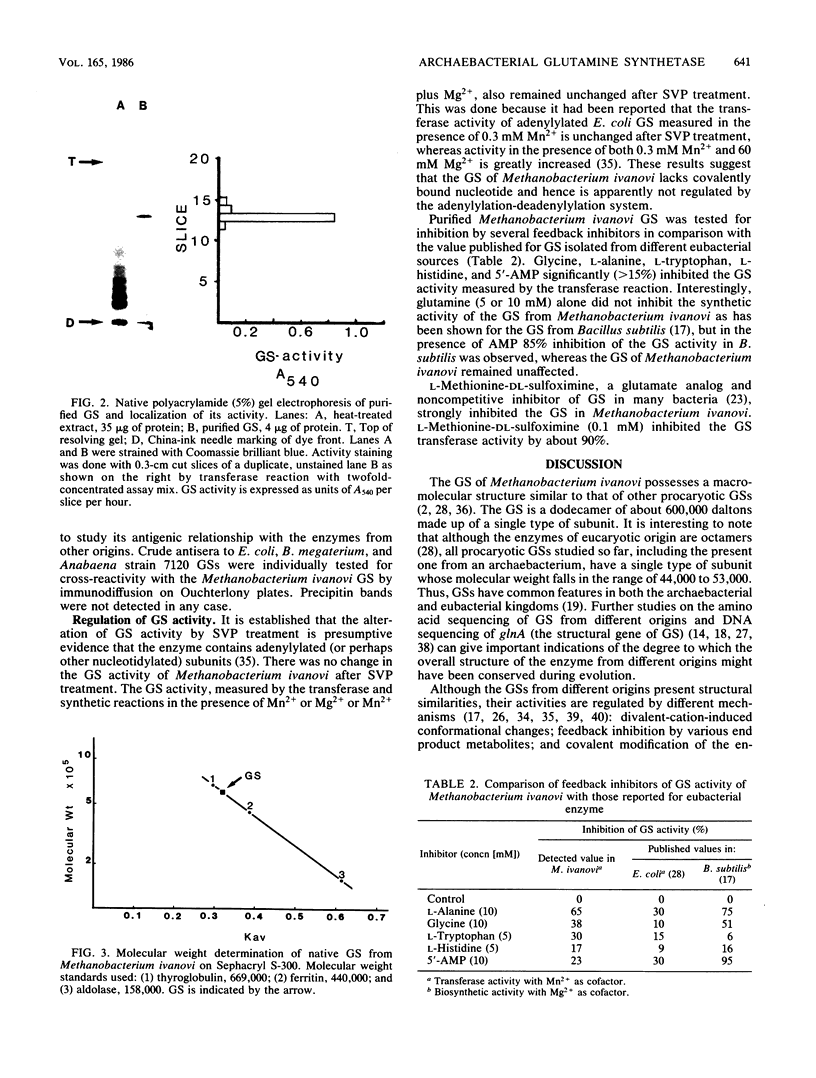
Glutamine synthetase (GS) was purified to electrophoretic homogeneity from the obligate anaerobic archaebacterium Methanobacterium ivanovi. The 130-fold-purified enzyme was obtained by heat treatment, ion-exchange chromatography, and gel filtration. Like all other eubacterial GSs known so far, the GS of M. ivanovi was found to be a dodecamer of about 600,000 daltons composed of a single type of subunit. The enzyme was stable at 63 degrees C for 10 min and was not sensitive to oxygen. The isoelectric point was 4.6, and the optimum pH of gamma-glutamyltransferase activity was 8.0. The Km values for hydroxylamine, glutamine, and ADP in the transferase reaction were 6.8, 22.7, and 0.35 mM, respectively. L-Methionine-DL-sulfoximine strongly inhibited the activity. Like the GS from gram-positive bacteria, Anabaena sp., several yeasts, and mammals, the enzyme from M. ivanovi was not regulated by adenylylation as demonstrated by snake venom phosphodiesterase treatment. Inhibition of the transferase activity by L-alanine, glycine, L-histidine, and L-tryptophan was observed. L-Glutamine alone or in the presence of AMP did not inhibit the GS synthetic activity. The GS of Methanobacterium ivanovi did not cross-react with a variety of antisera against GS from Escherichia coli, Anabaena strain 7120, or Bacillus megaterium. Archaebacterial GS appears to be structurally and functionally similar to eubacterial GS in gram-positive bacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. P., Purich D., Stadtman E. R. Cascade control of Escherichia coli glutamine synthetase. Properties of the PII regulatory protein and the uridylyltransferase-uridylyl-removing enzyme. J Biol Chem. 1975 Aug 25;250(16):6264–6272. [PubMed] [Google Scholar]
- Alef K., Burkardt H. J., Horstmann H. J., Zumft W. G. Molecular characterization of glutamine synthetase from the nitrogen-fixing phototrophic bacterium Rhodopseudomonas palustris. Z Naturforsch C. 1981 Mar-Apr;36(3-4):246–254. doi: 10.1515/znc-1981-3-411. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay N., Sparling R., Daniels L. Dinitrogen fixation by a thermophilic methanogenic bacterium. Nature. 1984 Nov 15;312(5991):286–288. doi: 10.1038/312286a0. [DOI] [PubMed] [Google Scholar]
- Belyaev S. S., Wolkin R., Kenealy W. R., Deniro M. J., Epstein S., Zeikus J. G. Methanogenic bacteria from the bondyuzhskoe oil field: general characterization and analysis of stable-carbon isotopic fractionation. Appl Environ Microbiol. 1983 Feb;45(2):691–697. doi: 10.1128/aem.45.2.691-697.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R. A., Janssen K. A., Resnick A. D., Blumenberg M., Foor F., Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977 Feb;129(2):1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar L., Jain M. K., Aubert J. P., Zeikus J. G. Comparison of assimilatory organic nitrogen, sulfur, and carbon sources for growth of methanobacterium species. Appl Environ Microbiol. 1984 Oct;48(4):785–790. doi: 10.1128/aem.48.4.785-790.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chock P. B., Rhee S. G., Stadtman E. R. Interconvertible enzyme cascades in cellular regulation. Annu Rev Biochem. 1980;49:813–843. doi: 10.1146/annurev.bi.49.070180.004121. [DOI] [PubMed] [Google Scholar]
- Covarrubias A. A., Bastarrachea F. Nucleotide sequence of the glnA control region of Escherichia coli. Mol Gen Genet. 1983;190(1):171–175. doi: 10.1007/BF00330342. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Daniels L., Sparling R., Sprott G. D. The bioenergetics of methanogenesis. Biochim Biophys Acta. 1984 Sep 6;768(2):113–163. doi: 10.1016/0304-4173(84)90002-8. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Prusiner S. Regulation of glutamine synthetase from Bacillus subtilis by divalent cations, feedback inhibitors, and L-glutamine. J Biol Chem. 1974 Jan 10;249(1):257–264. [PubMed] [Google Scholar]
- Fisher R., Tuli R., Haselkorn R. A cloned cyanobacterial gene for glutamine synthetase functions in Escherichia coli, but the enzyme is not adenylylated. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3393–3397. doi: 10.1073/pnas.78.6.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Kenealy W. R., Thompson T. E., Schubert K. R., Zeikus J. G. Ammonia assimilation and synthesis of alanine, aspartate, and glutamate in Methanosarcina barkeri and Methanobacterium thermoautotrophicum. J Bacteriol. 1982 Jun;150(3):1357–1365. doi: 10.1128/jb.150.3.1357-1365.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller R. E., Shelton E., Stadtman E. R. Zinc-induced paracrystalline aggregation of glutamine synthetase. Arch Biochem Biophys. 1974 Jul;163(1):155–171. doi: 10.1016/0003-9861(74)90465-2. [DOI] [PubMed] [Google Scholar]
- Orr J., Haselkorn R. Kinetic and inhibition studies of glutamine synthetase from the cyanobacterium Anabaena 7120. J Biol Chem. 1981 Dec 25;256(24):13099–13104. [PubMed] [Google Scholar]
- Orr J., Keefer L. M., Keim P., Nguyen T. D., Wellems T., Heinrikson R. L., Haselkorn R. Purification, physical characterization, and NH2-terminal sequence of glutamine synthetase from the cyanobacterium Anabaena 7120. J Biol Chem. 1981 Dec 25;256(24):13091–13098. [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Stacey G., Van Baalen C., Tabita F. R. Nitrogen and ammonia assimilation in the cyanobacteria: regulation of glutamine synthetase. Arch Biochem Biophys. 1979 May;194(2):457–467. doi: 10.1016/0003-9861(79)90640-4. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Ginsburg A., Ciardi J. E., Yeh J., Hennig S. B., Shapiro B. M. Multiple molecular forms of glutamine synthetase produced by enzyme catalyzed adenylation and deadenylylation reactions. Adv Enzyme Regul. 1970;8:99–118. doi: 10.1016/0065-2571(70)90011-7. [DOI] [PubMed] [Google Scholar]
- Streicher S. L., Tyler B. Purification of glutamine synthetase from a variety of bacteria. J Bacteriol. 1980 Apr;142(1):69–78. doi: 10.1128/jb.142.1.69-78.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick S. R., Ciardi J. E., Stadtman E. R. Comparative biochemical and immunological studies of bacterial glutamine synthetases. J Bacteriol. 1973 Sep;115(3):858–868. doi: 10.1128/jb.115.3.858-868.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B. Regulation of the assimilation of nitrogen compounds. Annu Rev Biochem. 1978;47:1127–1162. doi: 10.1146/annurev.bi.47.070178.005403. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]