Abstract
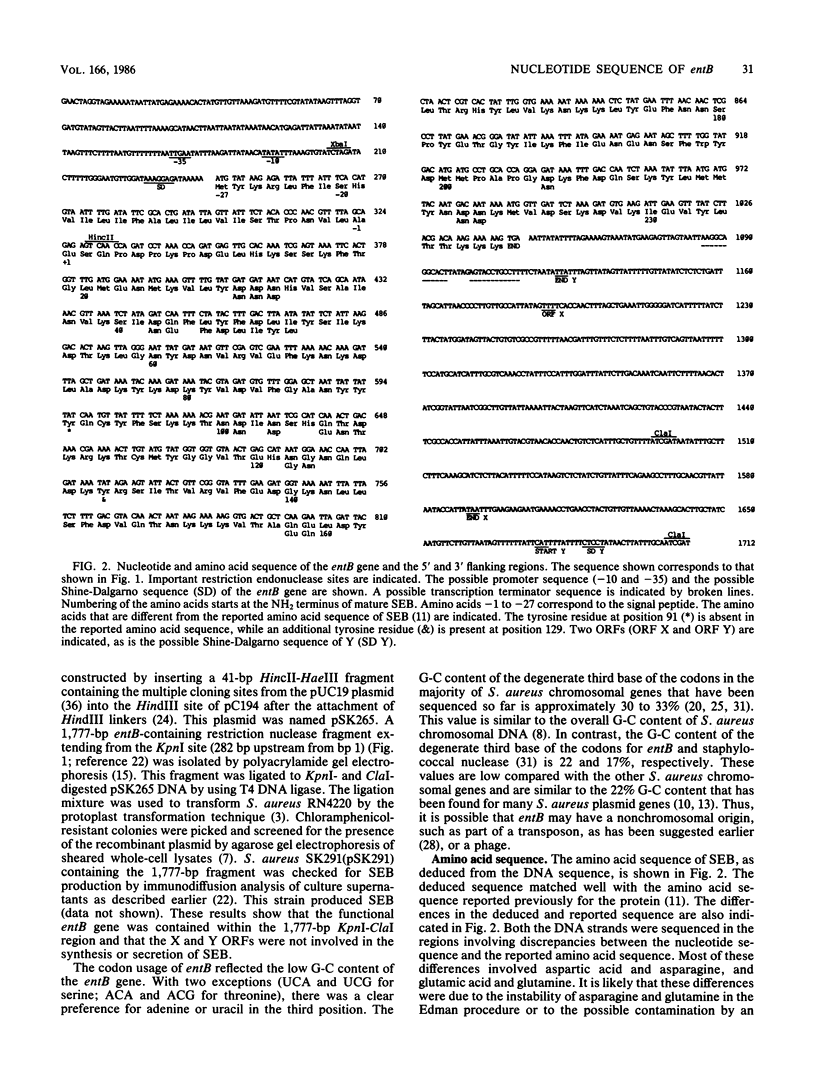
The complete nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus, as well as the 5' and 3' flanking regions, was determined. Starting from an ATG initiator codon, an open reading frame encoded the enterotoxin B precursor that consisted of 266 amino acids (Mr, 31,400). The 5' terminal portion of the gene encodes a signal peptide 27 amino acids long. The deduced amino acid sequence matched, with a few exceptions, the published amino acid sequence of enterotoxin B. The structural gene was flanked on the 5' side by a promoter-like sequence and on the 3' side by a palindromic structure followed by a thymine-rich region that resembled a transcription termination signal. Downstream from the entB gene were two overlapping open reading frames corresponding to 134 and 185 amino acids in the opposite orientation. The signal sequence of the enterotoxin B precursor resembled that of other secreted proteins found in other bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betley M. J., Löfdahl S., Kreiswirth B. N., Bergdoll M. S., Novick R. P. Staphylococcal enterotoxin A gene is associated with a variable genetic element. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5179–5183. doi: 10.1073/pnas.81.16.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalidowicz J. E., Silverman S. J., Schantz E. J., Stefanye D., Spero L. Chemical and biological properties of reduced and alkylated staphylococcal enterotoxin B. Biochemistry. 1966 Jul;5(7):2375–2381. doi: 10.1021/bi00871a029. [DOI] [PubMed] [Google Scholar]
- Dyer D. W., Iandolo J. J. Plasmid-chromosomal transition of genes important in staphylococcal enterotoxin B expression. Infect Immun. 1981 Aug;33(2):450–458. doi: 10.1128/iai.33.2.450-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I. Y., Bergdoll M. S. The primary structure of staphylococcal enterotoxin B. 3. The cyanogen bromide peptides of reduced and aminoethylated enterotoxin B, and the complete amino acid sequence. J Biol Chem. 1970 Jul 25;245(14):3518–3525. [PubMed] [Google Scholar]
- Khan S. A., Novick R. P. Complete nucleotide sequence of pT181, a tetracycline-resistance plasmid from Staphylococcus aureus. Plasmid. 1983 Nov;10(3):251–259. doi: 10.1016/0147-619x(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Khan S. A., Novick R. P. Structural analysis of plasmid pSN2 in Staphylococcus aureus: no involvement in enterotoxin B production. J Bacteriol. 1982 Feb;149(2):642–649. doi: 10.1128/jb.149.2.642-649.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Moreno F., Fowler A. V., Hall M., Silhavy T. J., Zabin I., Schwartz M. A signal sequence is not sufficient to lead beta-galactosidase out of the cytoplasm. Nature. 1980 Jul 24;286(5771):356–359. doi: 10.1038/286356a0. [DOI] [PubMed] [Google Scholar]
- Murphy E. Nucleotide sequence of ermA, a macrolide-lincosamide-streptogramin B determinant in Staphylococcus aureus. J Bacteriol. 1985 May;162(2):633–640. doi: 10.1128/jb.162.2.633-640.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Bergström S., Edlund T., Grundström T., Jaurin B., Lindberg F. P., Olsson O. Overlapping genes. Annu Rev Genet. 1983;17:499–525. doi: 10.1146/annurev.ge.17.120183.002435. [DOI] [PubMed] [Google Scholar]
- Ranelli D. M., Jones C. L., Johns M. B., Mussey G. J., Khan S. A. Molecular cloning of staphylococcal enterotoxin B gene in Escherichia coli and Staphylococcus aureus. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5850–5854. doi: 10.1073/pnas.82.17.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J., Lau L. F., Bahl C. P., Narang S. A., Wu R. Synthetic adaptors for cloning DNA. Methods Enzymol. 1979;68:98–109. doi: 10.1016/0076-6879(79)68009-6. [DOI] [PubMed] [Google Scholar]
- Sako T., Tsuchida N. Nucleotide sequence of the staphylokinase gene from Staphylococcus aureus. Nucleic Acids Res. 1983 Nov 25;11(22):7679–7693. doi: 10.1093/nar/11.22.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. J., Spero L. The complete amino acid sequence of staphylococcal enterotoxin C1. J Biol Chem. 1983 May 25;258(10):6300–6306. [PubMed] [Google Scholar]
- Schwindinger W. F., Warner J. R. DNA sequence analysis on the IBM-PC. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):601–604. doi: 10.1093/nar/12.1part2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Iandolo J. J. Genetics of staphylococcal enterotoxin B in methicillin-resistant isolates of Staphylococcus aureus. Infect Immun. 1979 Sep;25(3):902–911. doi: 10.1128/iai.25.3.902-911.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Iandolo J. J. Transduction of staphylococcal enterotoxin B synthesis: establishment of the toxin gene in a recombination-deficient mutant. Infect Immun. 1980 Jan;27(1):280–282. doi: 10.1128/iai.27.1.280-282.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalita Z., Hertman I., Sarid S. Isolation and characterization of a plasmid involved with enterotoxin B production in Staphylococcus aureus. J Bacteriol. 1977 Jan;129(1):317–325. doi: 10.1128/jb.129.1.317-325.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D. A genetic system for analysis of staphylococcal nuclease. Gene. 1983 May-Jun;22(2-3):181–189. doi: 10.1016/0378-1119(83)90102-6. [DOI] [PubMed] [Google Scholar]
- Spero L., Morlock B. A. Cross-reactions between tryptic polypeptides of staphylococcal enterotoxins B and C. J Immunol. 1979 Apr;122(4):1285–1289. [PubMed] [Google Scholar]
- Sugiyama H., Hayama T. Abdominal viscera as site of emetic action for staphylococcal enterotoxin in the monkey. J Infect Dis. 1965 Oct;115(4):330–336. doi: 10.1093/infdis/115.4.330. [DOI] [PubMed] [Google Scholar]
- Tweten R. K., Iandolo J. J. Purification and partial characterization of a putative precursor to staphylococcal enterotoxin B. Infect Immun. 1981 Dec;34(3):900–907. doi: 10.1128/iai.34.3.900-907.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlén M., Guss B., Nilsson B., Gatenbeck S., Philipson L., Lindberg M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J Biol Chem. 1984 Feb 10;259(3):1695–1702. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


