Abstract
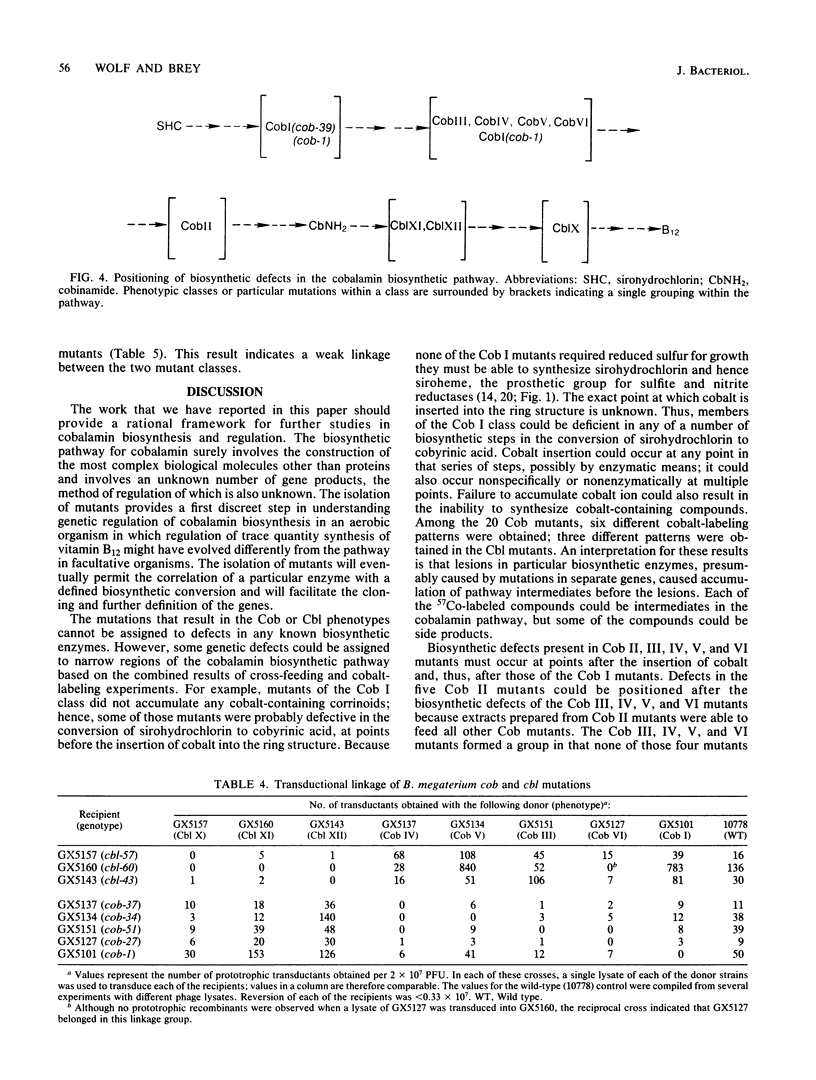
Ethanolamine is deaminated by the action of ethanolamine ammonia-lyase (EC 4.3.1.7), an adenosylcobalamin-dependent enzyme. Consequently, to grow on ethanolamine as a sole nitrogen source, Bacillus megaterium requires vitamin B12. Identification of B. megaterium mutants deficient for growth on ethanolamine as the sole nitrogen source yielded a total of 34 vitamin B12 auxotrophs. The vitamin B12 auxotrophs were divided into two major phenotypic groups: Cob mutants, which could use cobinamide or vitamin B12 to grow on ethanolamine, and Cbl mutants, which could be supplemented only by vitamin B12. The Cob mutants were resolved into six classes and the Cbl mutants were resolved into three, based on the spectrum of cobalt-labeled corrinoid compounds which they accumulated. Although some radiolabeled cobalamin was detected in the wild type, little or none was evident in the auxotrophs. The results indicate that Cob mutants contain lesions in biosynthetic steps before the synthesis of combinamide, while Cbl mutants are defective in the conversion of cobinamide to cobalamin. Analysis of phage-mediated transduction experiments revealed tight genetic linkage within the Cob class and within the Cbl class. Similar transduction analysis indicated the Cob and Cbl classes are weakly linked. In addition, cross-feeding experiments in which extracts prepared from mutants were examined for their effect on growth of various other mutants allowed a partial ordering of mutations within the cobalamin biosynthetic pathway.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAY R. C., SHEMIN D. On the biosynthesis of vitamin B-12. The derivation of the corrin structure from 5-aminolevulinic acid and the methyl group of methionine. J Biol Chem. 1963 Apr;238:1501–1508. [PubMed] [Google Scholar]
- Barker H. A. Corrinoid-dependent enzymic reactions. Annu Rev Biochem. 1972;41:55–90. doi: 10.1146/annurev.bi.41.070172.000415. [DOI] [PubMed] [Google Scholar]
- Blackwell C. M., Scarlett F. A., Turner J. M. Microbial metabolism of amino alcohols. Control of formation and stability of partially purified ethanolamine ammonia-lyase in Escherichia coli. J Gen Microbiol. 1977 Jan;98(1):133–139. doi: 10.1099/00221287-98-1-133. [DOI] [PubMed] [Google Scholar]
- Blackwell C. M., Turner J. M. Microbial metabolism of amino alcohols. Formation of coenzyme B12-dependent ethanolamine ammonia-lyase and its concerted induction in Escherichia coli. Biochem J. 1978 Dec 15;176(3):751–757. doi: 10.1042/bj1760751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell C. M., Turner J. M. Microbial metabolism of amino alcohols. Purification and properties of coenzyme B12-dependent ethanolamine ammonia-lyase of Escherichia coli. Biochem J. 1978 Nov 1;175(2):555–563. doi: 10.1042/bj1750555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbeer C. The clostridial fermentations of choline and ethanolamine. 1. Preparation and properties of cell-free extracts. J Biol Chem. 1965 Dec;240(12):4669–4674. [PubMed] [Google Scholar]
- Bradbeer C. The clostridial fermentations of choline and ethanolamine. II. Requirement for a cobamide coenzyme by an ethanolamine deaminase. J Biol Chem. 1965 Dec;240(12):4675–4681. [PubMed] [Google Scholar]
- Brown B. J., Carlton B. C. Plasmid-mediated transformation in Bacillus megaterium. J Bacteriol. 1980 May;142(2):508–512. doi: 10.1128/jb.142.2.508-512.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G. W., Chang J. T. Evidence for the B12-dependent enzyme ethanolamine deaminase in Salmonella. Nature. 1975 Mar 13;254(5496):150–151. doi: 10.1038/254150a0. [DOI] [PubMed] [Google Scholar]
- Hogenkamp H. P. Chemical synthesis and properties of analogs of adenosylcobalamin. Biochemistry. 1974 Jun 18;13(13):2736–2740. doi: 10.1021/bi00710a012. [DOI] [PubMed] [Google Scholar]
- Jackson R. H., Cornish-Bowden A., Cole J. A. Prosthetic groups of the NADH-dependent nitrite reductase from Escherichia coli K12. Biochem J. 1981 Mar 1;193(3):861–867. doi: 10.1042/bj1930861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter R. M., Olivera B. M., Roth J. R. Salmonella typhimurium synthesizes cobalamin (vitamin B12) de novo under anaerobic growth conditions. J Bacteriol. 1984 Jul;159(1):206–213. doi: 10.1128/jb.159.1.206-213.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan B. H., Stadtman E. R. Ethanolamine deaminase, a cobamide coenzyme-dependent enzyme. I. Purification, assay, and properties of the enzyme. J Biol Chem. 1968 Apr 25;243(8):1787–1793. [PubMed] [Google Scholar]
- Sando G. N., Grant M. E., Hogenkamp H. P. The interaction of adeninylalkylcobalamins with ribonucleotide reductase. Biochim Biophys Acta. 1976 Mar 25;428(1):228–232. doi: 10.1016/0304-4165(76)90123-9. [DOI] [PubMed] [Google Scholar]
- Scarlett F. A., Turner J. M. Microbial metabolism of amino alcohols. Ethanolamine catabolism mediated by coenzyme B12-dependent ethanolamine ammonia-lyase in Escherichia coli and Klebsiella aerogenes. J Gen Microbiol. 1976 Jul;95(1):173–176. doi: 10.1099/00221287-95-1-173. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Davis P. S., Murphy M. J. Incorporation of methionine-derived methyl groups into sirohaem by Escherichia coli. Biochem J. 1977 Dec 1;167(3):669–674. doi: 10.1042/bj1670669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary P. S., Garbe J. C., Franzen M., Frampton E. W. MP13, a generalized transducing bacteriophage for Bacillus megaterium. J Bacteriol. 1982 Mar;149(3):1112–1119. doi: 10.1128/jb.149.3.1112-1119.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]




