Abstract
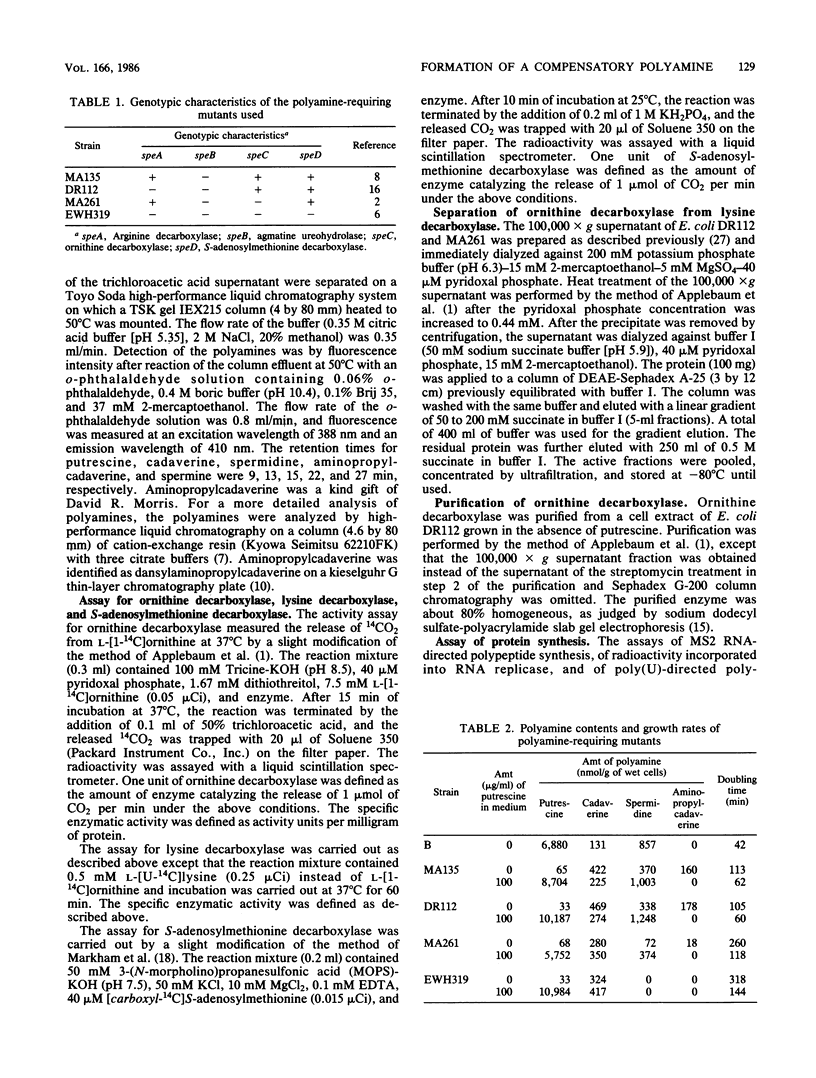
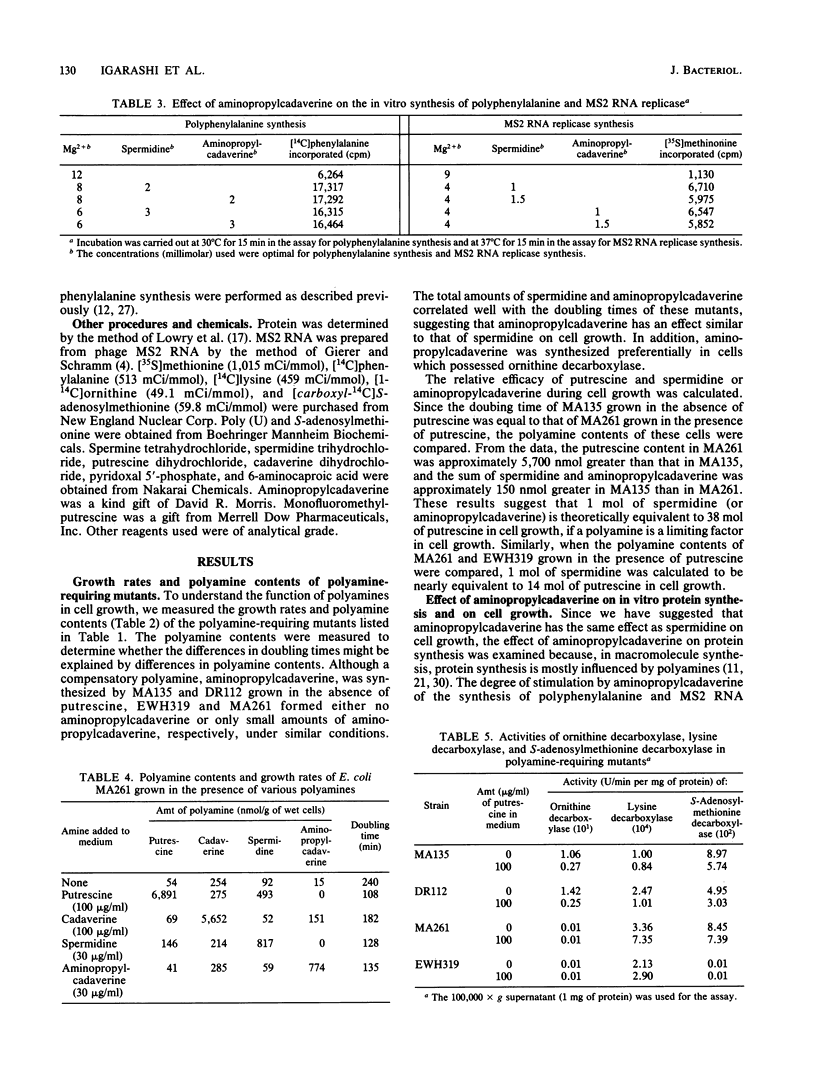
The amounts of normal and compensatory polyamines of polyamine-requiring Escherichia coli mutants grown in the absence of polyamines were determined. Although aminopropylcadaverine, a compensatory polyamine, was synthesized by MA135 (speB) and DR112 (speA speB), no aminopropylcadaverine or only small amounts of aminopropylcadaverine were synthesized by EWH319 (speA speB speC speD) and MA261 (speB speC), respectively. The average mass doubling times of MA135, DR112, MA261, and EWH319 grown in the absence of polyamines were 113, 105, 260, and 318 min, respectively. The correlation of these values with the sum of spermidine plus aminopropylcadaverine suggested that aminopropylcadaverine is important for cell growth in the presence of limiting amounts of normal polyamines. This hypothesis is supported by the results of aminopropylcadaverine stimulation of the in vitro synthesis of polyphenylalanine and MS2 RNA replicase and of its stimulation of the growth of MA261. For the following reasons, it was concluded that aminopropylcadaverine was synthesized preferentially from cadaverine made by ornithine decarboxylase: aminopropylcadaverine was synthesized in relatively large amounts in cells (MA135 and DR112) which possess ornithine decarboxylase; ornithine decarboxylase catalyzed the decarboxylation of lysine in vitro, and the in vivo formation of aminopropylcadaverine was inhibited by an inhibitor of ornithine decarboxylase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebaum D. M., Dunlap J. C., Morris D. R. Comparison of the biosynthetic and biodegradative ornithine decarboxylases of Escherichia coli. Biochemistry. 1977 Apr 19;16(8):1580–1584. doi: 10.1021/bi00627a008. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles S., Maas W. K. Isolation, characterization, and mapping of Escherichia coli mutants blocked in the synthesis of ornithine decarboxylase. J Bacteriol. 1975 Nov;124(2):791–799. doi: 10.1128/jb.124.2.791-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamine stimulation of nucleic acid synthesis in an uninfected and phage-infected polyamine auxotroph of Escherichia coli K12 (arginine-agmatine ureohydrolase-putrescine-spermidine-lysine-cadaverine). Proc Natl Acad Sci U S A. 1972 Jan;69(1):213–217. doi: 10.1073/pnas.69.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIERER A., SCHRAMM G. Infectivity of ribonucleic acid from tobacco mosaic virus. Nature. 1956 Apr 14;177(4511):702–703. doi: 10.1038/177702a0. [DOI] [PubMed] [Google Scholar]
- Goldemberg S. H. Lysine decarboxylase mutants of Escherichia coli: evidence for two enzyme forms. J Bacteriol. 1980 Mar;141(3):1428–1431. doi: 10.1128/jb.141.3.1428-1431.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner E. W., Tabor C. W., Tabor H. Mutants of Escherichia coli that do not contain 1,4-diaminobutane (putrescine) or spermidine. J Biol Chem. 1979 Dec 25;254(24):12419–12426. [PubMed] [Google Scholar]
- Hamana K., Matsuzaki S. Unusual polyamines in slime molds Physarum polycephalum and Dictyostelium discoideum. J Biochem. 1984 Apr;95(4):1105–1110. doi: 10.1093/oxfordjournals.jbchem.a134698. [DOI] [PubMed] [Google Scholar]
- Hirshfield I. N., Rosenfeld H. J., Leifer Z., Maas W. K. Isolation and characterization of a mutant of Escherichia coli blocked in the synthesis of putrescine. J Bacteriol. 1970 Mar;101(3):725–730. doi: 10.1128/jb.101.3.725-730.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Morris D. R. Physiological effects in bovine lymphocytes of inhibiting polyamine synthesis with ethylglyoxal bis(guanylhydrazone). Cancer Res. 1984 Nov;44(11):5332–5337. [PubMed] [Google Scholar]
- Igarashi K., Sugawara K., Izumi I., Nagayama C., Hirose S. Effect of polyamines of polyphenylalanine synthesis by Escherichia coli and rat-liver ribosomes. Eur J Biochem. 1974 Oct 2;48(2):495–502. doi: 10.1111/j.1432-1033.1974.tb03790.x. [DOI] [PubMed] [Google Scholar]
- Jorstad C. M., Harada J. J., Morris D. R. Structural specificity of the spermidine requirement of an Escherichia coli auxotroph. J Bacteriol. 1980 Feb;141(2):456–463. doi: 10.1128/jb.141.2.456-463.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio A., McCann P. P., Bey P. DL-a-Monofluoromethylputrescine is a potent irreversible inhibitor of Escherichia coli ornithine decarboxylase. Biochem J. 1982 Jun 15;204(3):771–775. doi: 10.1042/bj2040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linderoth N., Morris D. R. Structural specificity of the triamines sym-homospermidine and aminopropylcadaverine in stimulating growth of spermidine auxotrophs of Escherichia coli. Biochem Biophys Res Commun. 1983 Dec 16;117(2):616–622. doi: 10.1016/0006-291x(83)91245-7. [DOI] [PubMed] [Google Scholar]
- Markham G. D., Tabor C. W., Tabor H. S-adenosylmethionine decarboxylase (Escherichia coli). Methods Enzymol. 1983;94:228–230. doi: 10.1016/s0076-6879(83)94039-9. [DOI] [PubMed] [Google Scholar]
- Paulus T. J., Kiyono P., Davis R. H. Polyamine-deficient Neurospora crassa mutants and synthesis of cadaverine. J Bacteriol. 1982 Oct;152(1):291–297. doi: 10.1128/jb.152.1.291-297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., McGill S. Decarboxylation of ornithine and lysine in rat tissues. Biochim Biophys Acta. 1979 Jun 6;568(2):416–427. doi: 10.1016/0005-2744(79)90310-3. [DOI] [PubMed] [Google Scholar]
- Rudkin B. B., Mamont P. S., Seiler N. Decreased protein-synthetic activity is an early consequence of spermidine depletion in rat hepatoma tissue-culture cells. Biochem J. 1984 Feb 1;217(3):731–741. doi: 10.1042/bj2170731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabo D. L., Boeker E. A., Byers B., Waron H., Fischer E. H. Purification and physical properties of inducible Escherichia coli lysine decarboxylase. Biochemistry. 1974 Feb 12;13(4):662–670. doi: 10.1021/bi00701a005. [DOI] [PubMed] [Google Scholar]
- Sabo D. L., Fischer E. H. Chemical properties of Escherichia coli lysine decarboxylase including a segment of its pyridoxal 5'-phosphate binding site. Biochemistry. 1974 Feb 12;13(4):670–676. doi: 10.1021/bi00701a006. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tabor H., Hafner E. W., Tabor C. W. Construction of an Escherichia coli strain unable to synthesize putrescine, spermidine, or cadaverine: characterization of two genes controlling lysine decarboxylase. J Bacteriol. 1980 Dec;144(3):952–956. doi: 10.1128/jb.144.3.952-956.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Igarashi K., Hirose S. Differential stimulation by polyamines of phage RNA-directed synthesis of proteins. Biochim Biophys Acta. 1981 Dec 28;656(2):134–139. doi: 10.1016/0005-2787(81)90078-2. [DOI] [PubMed] [Google Scholar]
- Wertheimer S. J., Leifer Z. Putrescine and spermidine sensitivity of lysine decarboxylase in Escherichia coli: evidence for a constitutive enzyme and its mode of regulation. Biochem Biophys Res Commun. 1983 Jul 29;114(2):882–888. doi: 10.1016/0006-291x(83)90863-x. [DOI] [PubMed] [Google Scholar]
- Young D. V., Srinivasan P. R. Growth of ribonucleic acid bacteriophage f2 in a conditional putrescine auxotroph of Escherichia coli: evidence for a polyamine role in translation. J Bacteriol. 1974 Mar;117(3):1280–1288. doi: 10.1128/jb.117.3.1280-1288.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


