Abstract
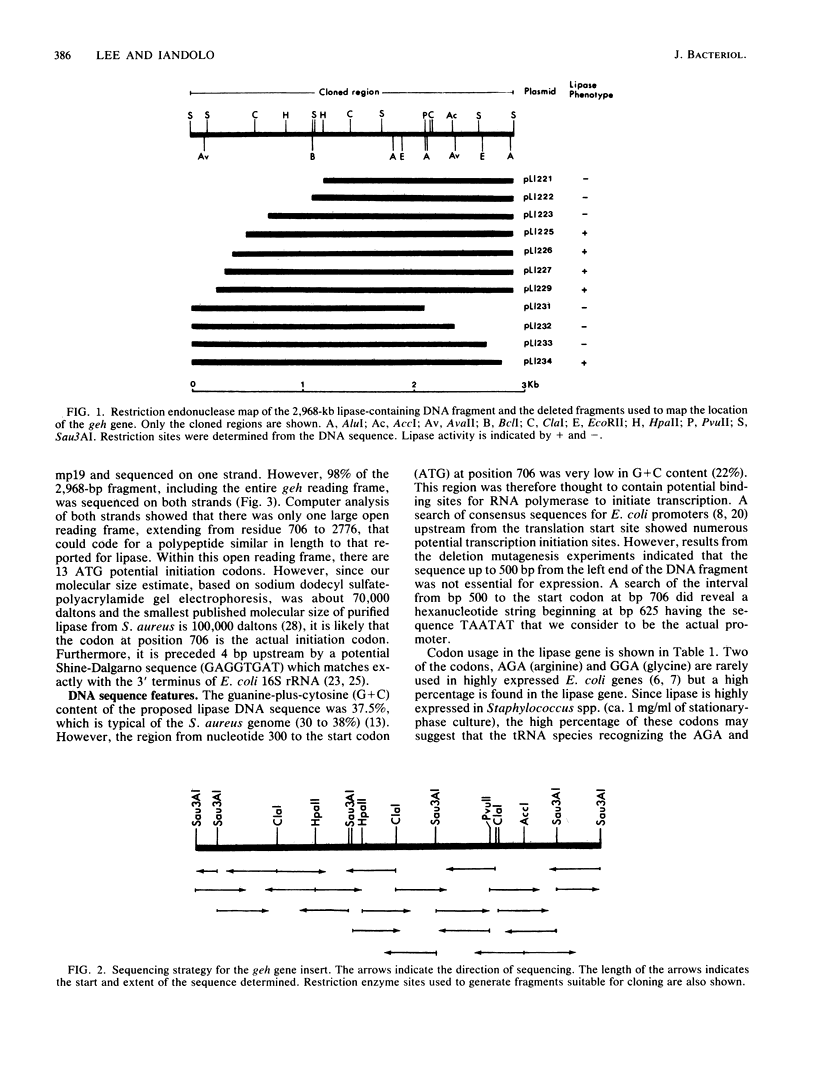
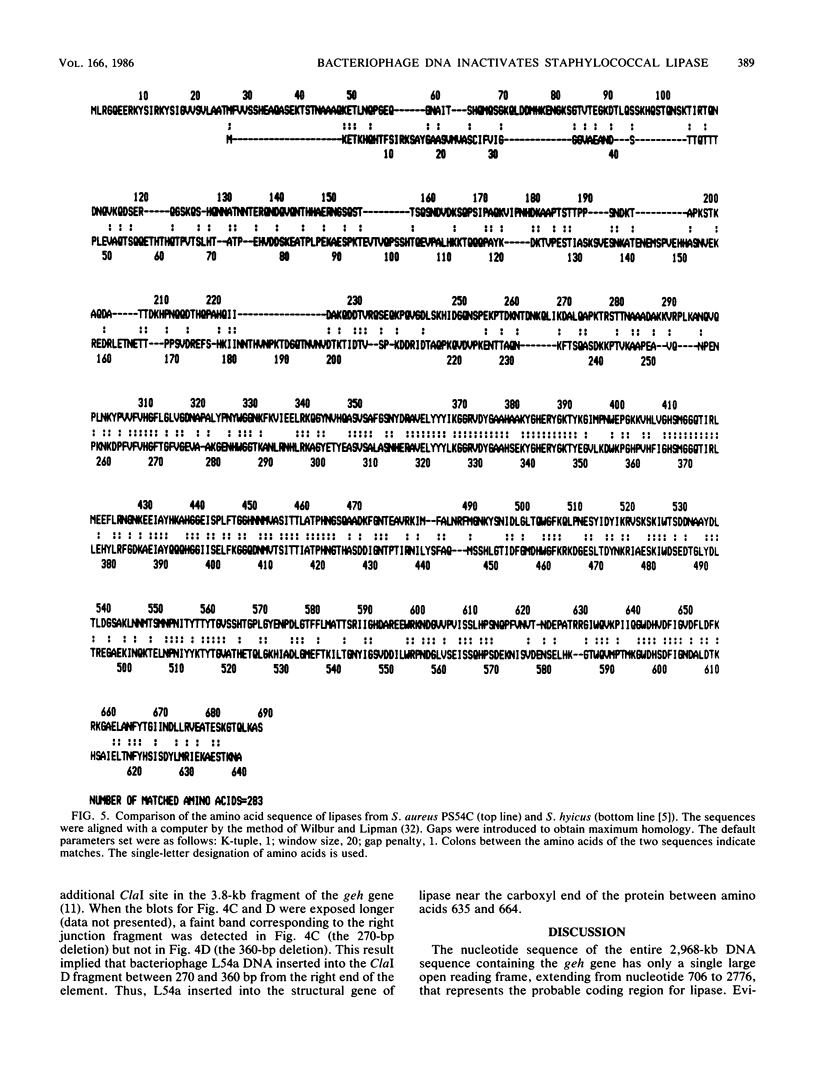
Staphylococcus aureus PS54 manifests no lipase (geh) activity. This is due to the insertion of bacteriophage L54a DNA into the geh structural gene. The nucleotide sequence of this 2,968-base-pair DNA fragment was determined. Lipase deduced from the nucleotide sequence is a polypeptide of 690 amino acids which extends from nucleotide 706 to 2776.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Duval-Iflah Y. Lysogenic conversion of the lipase in Staphylococcus pyogenes group 3 strains. Can J Microbiol. 1972 Sep;18(9):1491–1497. doi: 10.1139/m72-228. [DOI] [PubMed] [Google Scholar]
- Dyer D. W., Rock M. I., Iandolo J. J. Autogenous transduction of phi 11de in Staphylococcus aureus: transfer and genetic properties. J Bacteriol. 1984 May;158(2):689–695. doi: 10.1128/jb.158.2.689-695.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Götz F., Popp F., Korn E., Schleifer K. H. Complete nucleotide sequence of the lipase gene from Staphylococcus hyicus cloned in Staphylococcus carnosus. Nucleic Acids Res. 1985 Aug 26;13(16):5895–5906. doi: 10.1093/nar/13.16.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes W. M., Platt T., Rosenberg M. Termination of transcription in E. coli. Cell. 1983 Apr;32(4):1029–1032. doi: 10.1016/0092-8674(83)90287-8. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Iandolo J. J. Mechanism of bacteriophage conversion of lipase activity in Staphylococcus aureus. J Bacteriol. 1985 Oct;164(1):288–293. doi: 10.1128/jb.164.1.288-293.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates A. Characterization and properties of purified Staphylococcus aureus lipase. Microbios. 1974 Jan;9(33):61–74. [PubMed] [Google Scholar]
- POLLOCK M. R., RICHMOND M. H. Low cyst(e)ine content of bacterial extracellular proteins: its possible physiological significance. Nature. 1962 May 5;194:446–449. doi: 10.1038/194446a0. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Ranelli D. M., Jones C. L., Johns M. B., Mussey G. J., Khan S. A. Molecular cloning of staphylococcal enterotoxin B gene in Escherichia coli and Staphylococcus aureus. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5850–5854. doi: 10.1073/pnas.82.17.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. D., Roufa D. J. Emetine resistance of Chinese hamster cells: structures of wild-type and mutant ribosomal protein S14 mRNAs. Mol Cell Biol. 1985 Jul;5(7):1655–1659. doi: 10.1128/mcb.5.7.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rosendal K., Bülow P. Temperate phages influencing lipase production by Staphylococcus aureus. J Gen Microbiol. 1965 Dec;41(3):349–356. doi: 10.1099/00221287-41-3-349. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweten R. K., Iandolo J. J. Transport and processing of staphylococcal enterotoxin B. J Bacteriol. 1983 Jan;153(1):297–303. doi: 10.1128/jb.153.1.297-303.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadehra D. V., Harmon L. G. Characterization of purified staphylococcal lipase. Appl Microbiol. 1967 May;15(3):480–483. doi: 10.1128/am.15.3.480-483.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadehra D. V., Harmon L. G. Isolation and purification of staphylococcal lipase. Appl Microbiol. 1967 Mar;15(2):292–295. doi: 10.1128/am.15.2.292-295.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger R. Enzyme kinetics of lipolysis. Methods Enzymol. 1980;64:340–392. doi: 10.1016/s0076-6879(80)64016-6. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]