Abstract
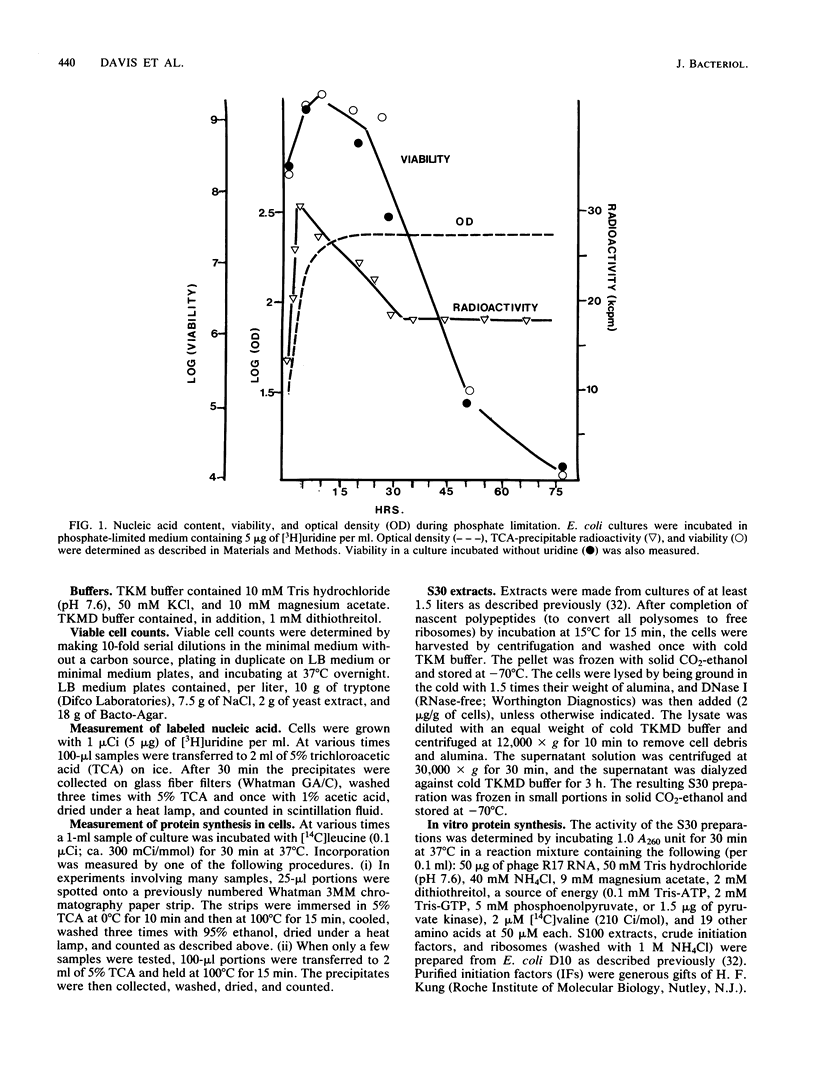
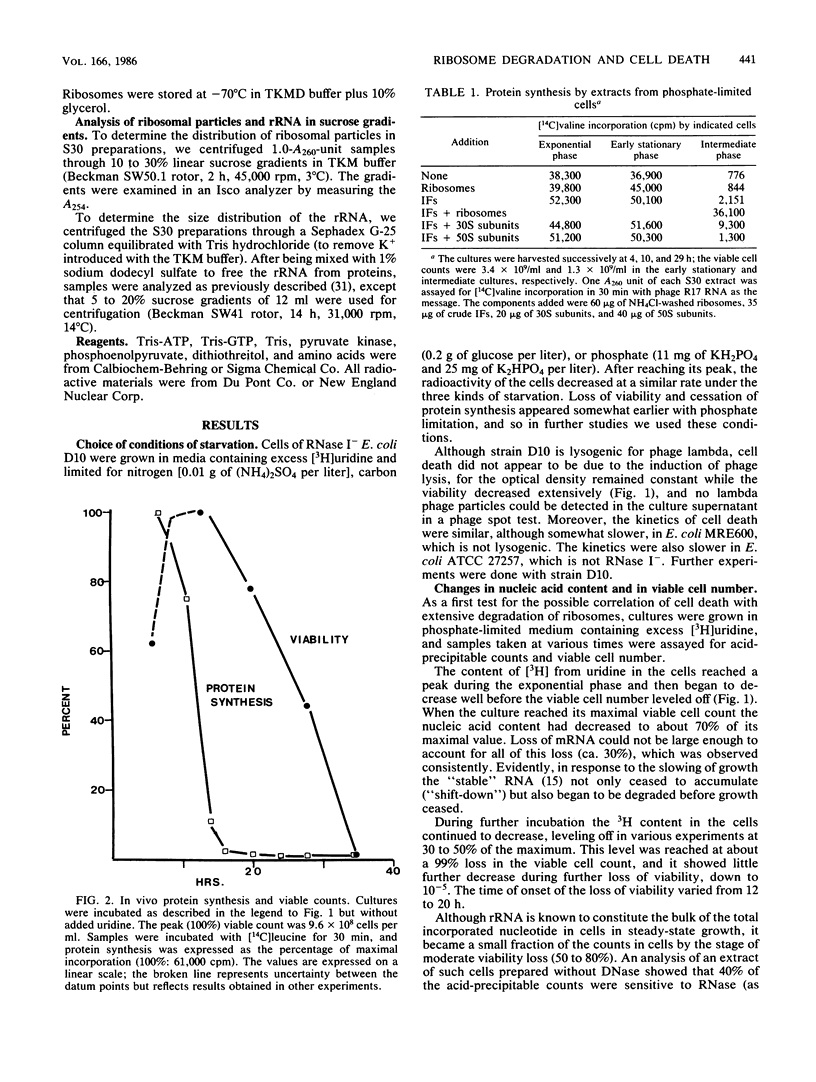
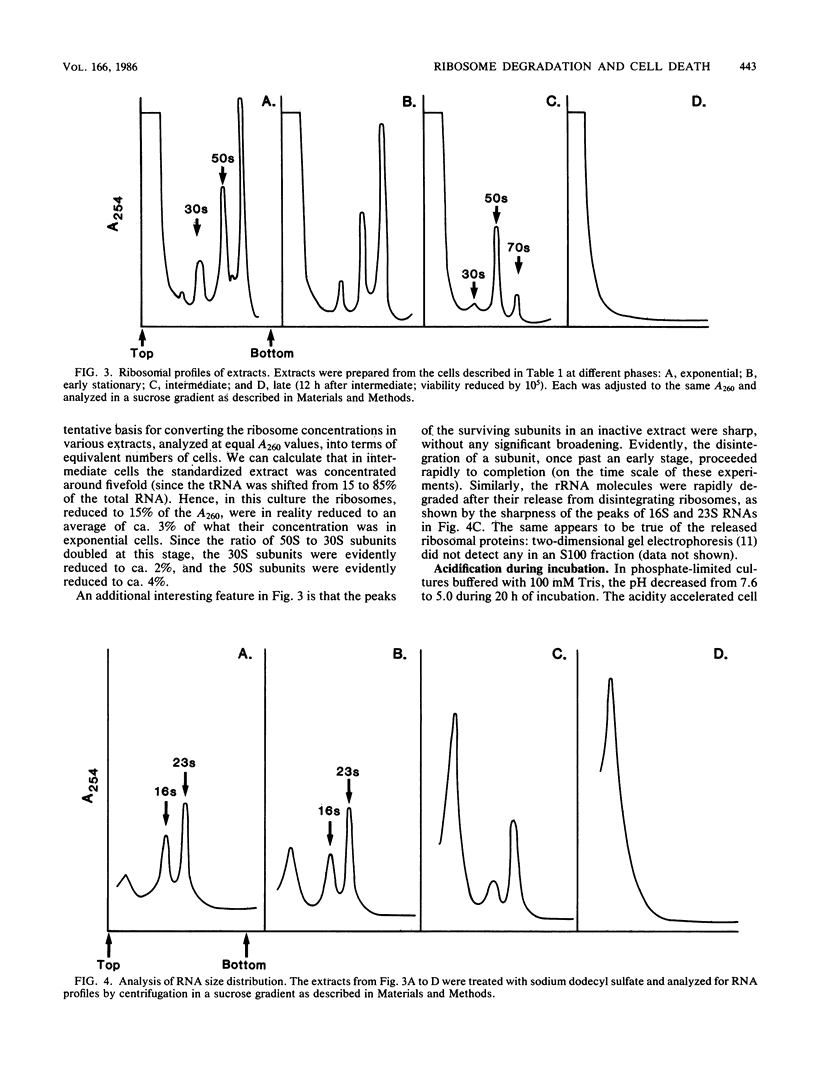
In Escherichia coli cultures limited for phosphate, the number of ribosomal particles was reduced to a small percentage of its earlier peak value by the time the viable cell count began to drop; the 30S subunits decreased more than the 50S subunits. Moreover, the ribosomal activity was reduced even more: these cells no longer synthesized protein, and their extracts could not translate phage RNA unless ribosomes were added. The translation initiation factors also disappeared, suggesting that they become less stable when released from their normal attachment to 30S subunits. In contrast, elongation factors, aminoacyl-tRNA synthetases, and tRNA persisted. During further incubation, until viability was reduced to 10(-5), the ribosomal particles disappeared altogether, while tRNA continued to be preserved. These results suggest that an excessive loss of ribosomes (and of initiation factors) may be a major cause of cell death during prolonged phosphate starvation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonowitz Y., Ron E. Z. Translocation in Bacillus subtilis: irreversible inactivation of elongation factor G and viability of a temperature- sensitive mutant. J Bacteriol. 1976 Mar;125(3):1229–1231. doi: 10.1128/jb.125.3.1229-1231.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hamida F., Schlessinger D. Synthesis and breakdown of ribonucleic acid in Escherichia coli starving for nitrogen. Biochim Biophys Acta. 1966 Apr 18;119(1):183–191. doi: 10.1016/0005-2787(66)90049-9. [DOI] [PubMed] [Google Scholar]
- Caulfield M. P., Horiuchi S., Tai P. C., Davis B. D. The 64-kilodalton membrane protein of Bacillus subtilis is also present as a multiprotein complex on membrane-free ribosomes. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7772–7776. doi: 10.1073/pnas.81.24.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. SOME ASPECTS OF THE ENDOGENOUS METABOLISM OF BACTERIA. Bacteriol Rev. 1964 Jun;28:126–149. doi: 10.1128/br.28.2.126-149.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. STUDIES ON THE ENDOGENOUS METABOLISM OF ESCHERICHIA COLI. Biochem J. 1965 May;95:332–343. doi: 10.1042/bj0950332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P. Synthesis and stability of individual ribosomal proteins in the presence of rifampicin. Mol Gen Genet. 1974;134(1):39–47. doi: 10.1007/BF00332811. [DOI] [PubMed] [Google Scholar]
- Fallon A. M., Jinks C. S., Yamamoto M., Nomura M. Expression of ribosomal protein genes cloned in a hybrid plasmid in Escherichia coli: gene dosage effects on synthesis of ribosomal proteins and ribosomal protein messenger ribonucleic acid. J Bacteriol. 1979 May;138(2):383–396. doi: 10.1128/jb.138.2.383-396.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furano A. V. Content of elongation factor Tu in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4780–4784. doi: 10.1073/pnas.72.12.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Geyl D., Böck A., Isono K. An improved method for two-dimensional gel-electrophoresis: analysis of mutationally altered ribosomal proteins of Escherichia coli. Mol Gen Genet. 1981;181(3):309–312. doi: 10.1007/BF00425603. [DOI] [PubMed] [Google Scholar]
- Kaplan R., Apirion D. Decay of ribosomal ribonucleic acid in Escherichia coli cells starved for various nutrients. J Biol Chem. 1975 Apr 25;250(8):3174–3178. [PubMed] [Google Scholar]
- Kaplan R., Apirion D. The fate of ribosomes in Escherichia coli cells starved for a carbon source. J Biol Chem. 1975 Mar 10;250(5):1854–1863. [PubMed] [Google Scholar]
- MALLETTE M. F., COWAN C. I., CAMPBELL J. J. GROWTH AND SURVIVAL OF ESCHERICHIA COLI IN MEDIUM LIMITED IN PHOSPHATE. J Bacteriol. 1964 Apr;87:779–785. doi: 10.1128/jb.87.4.779-785.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama H. B., Okamura S. Ribosome degradation and the degradation products in starved Escherichia coli. V. Ribonucleoprotein particles from glucose-starved cells. J Bacteriol. 1972 Apr;110(1):442–446. doi: 10.1128/jb.110.1.442-446.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama H., Mizuno D. Ribosome degradation and the degradation products in starved Escherichia coli. I. Comparison of the degradation rate and of the nucleotide pool between Escherichia coli B and Q-13 strains in phosphate deficiency. Biochim Biophys Acta. 1970 Jan 21;199(1):159–165. [PubMed] [Google Scholar]
- Maruyama H., Mizuno D. The participation of ribonuclease in the degradation of Escherichia coli ribosomal ribonucleic acid as revealed by oligonucleotides accumulation in the phorphorus-deficient stage. Biochim Biophys Acta. 1965 Dec 9;108(4):593–604. doi: 10.1016/0005-2787(65)90056-0. [DOI] [PubMed] [Google Scholar]
- Montague M. D., Dawes E. A. The survival of Peptococcus prévotii in relation to the adenylate energy charge. J Gen Microbiol. 1974 Jan;80(1):291–299. doi: 10.1099/00221287-80-1-291. [DOI] [PubMed] [Google Scholar]
- Nath K., Koch A. L. Protein degradation in Escherichia coli. II. Strain differences in the degradation of protein and nucleic acid resulting from starvation. J Biol Chem. 1971 Nov 25;246(22):6956–6967. [PubMed] [Google Scholar]
- Nauman R. K., Silverman D. J., Voelz H. Ribosomal helices: formation in Escherichia coli during acidic growth. J Bacteriol. 1971 Jul;107(1):358–360. doi: 10.1128/jb.107.1.358-360.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura S., Maruyama H. B., Yanagita T. Ribosome degradation and degradation products in starved Escherichia coli. VI. Prolonged culture during glucose starvation. J Biochem. 1973 May;73(5):915–922. doi: 10.1093/oxfordjournals.jbchem.a130174. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., WADE H. E., NESS A. G. The catabolism of proteins and nucleic acids in starved Aerobacter aerogenes. Biochem J. 1963 Feb;86:197–203. doi: 10.1042/bj0860197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John A. C., Goldberg A. L. Effects of starvation for potassium and other inorganic ions on protein degradation and ribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1980 Sep;143(3):1223–1233. doi: 10.1128/jb.143.3.1223-1233.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Ingraham J. L. A ribosome-like particle accumulated at low temperature by a cold-sensitive mutant of Salmonella typhimurium. Biochim Biophys Acta. 1971 Feb 25;232(1):151–166. doi: 10.1016/0005-2787(71)90499-0. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Wallace B. J., Herzog E. L., Davis B. D. Properties of initiation-free polysomes of Escherichia coli. Biochemistry. 1973 Feb;12(4):609–615. doi: 10.1021/bi00728a007. [DOI] [PubMed] [Google Scholar]
- Tal M., Silberstein A., Moyner K. In vivo reassembly of 30-S ribosomal subunits following their specific destruction by thermal shock. Biochim Biophys Acta. 1977 Dec 14;479(4):479–496. doi: 10.1016/0005-2787(77)90041-7. [DOI] [PubMed] [Google Scholar]


