Abstract
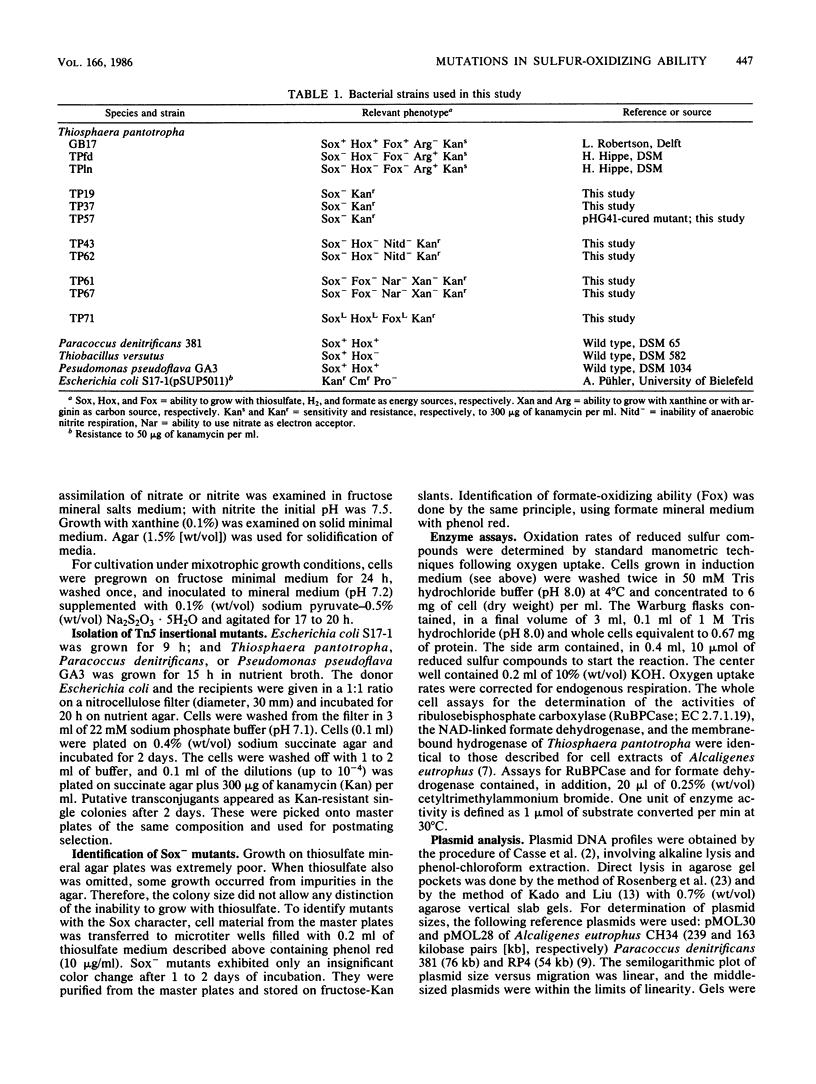
Mutants of Thiosphaera pantotropha defective in chemolithoautotrophic growth were obtained by transpositional mutagenesis with Tn5 coding for kanamycin resistance. The suicide vehicle for introducing Tn5 to T. pantotropha was pSUP5011 harbored by Escherichia coli. Kanamycin-resistant isolates were screened for the inability to grow with reduced sulfur compounds (Sox-). Four classes of Sox- mutants were obtained. Three were of different pleiotropic phenotypes: (i) unable to grow with formate, nitrate, and xanthine; (this class strongly suggested the involvement of a molybdenum cofactor in inorganic sulfur-oxidizing ability); (ii) no growth with hydrogen; (iii) slight growth with hydrogen and formate. Two plasmids, pHG41 (about 450 kilobase pairs) and pHG42 (110 kilobases), were identified in lysates of T. pantotropha. In one Sox- mutant pHG41 could not be detected. Revertant analysis suggested that pHG41 and pHG42 were not involved in the Sox character.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davidson M. S., Roy P., Summers A. O. Transpositional Mutagenesis of Thiobacillus novellus and Thiobacillus versutus. Appl Environ Microbiol. 1985 Jun;49(6):1436–1441. doi: 10.1128/aem.49.6.1436-1441.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M. S., Summers A. O. Wide-host-range plasmids function in the genus thiobacillus. Appl Environ Microbiol. 1983 Sep;46(3):565–572. doi: 10.1128/aem.46.3.565-572.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Hogrefe C., Schlegel H. G. Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J Bacteriol. 1981 Jul;147(1):198–205. doi: 10.1128/jb.147.1.198-205.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogrefe C., Römermann D., Friedrich B. Alcaligenes eutrophus hydrogenase genes (Hox). J Bacteriol. 1984 Apr;158(1):43–48. doi: 10.1128/jb.158.1.43-48.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im D. S., Friedrich C. G. Fluoride, hydrogen, and formate activate ribulosebisphosphate carboxylase formation in Alcaligenes eutrophus. J Bacteriol. 1983 May;154(2):803–808. doi: 10.1128/jb.154.2.803-808.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. P. Physiology of the thiobacilli: elucidating the sulphur oxidation pathway. Microbiol Sci. 1985;2(4):105–109. [PubMed] [Google Scholar]
- Kobayashi H., Akazawa T. Biosynthetic mechanism of ribulose-1,5-bisphosphate carboxylase in the purple photosynthetic bacterium, Chromatium vinosum. Arch Biochem Biophys. 1982 Apr 1;214(2):531–539. doi: 10.1016/0003-9861(82)90057-1. [DOI] [PubMed] [Google Scholar]
- PATEMAN J. A., COVE D. J., REVER B. M., ROBERTS D. B. A COMMON CO-FACTOR FOR NITRATE REDUCTASE AND XANTHINE DEHYDROGENASE WHICH ALSO REGULATES THE SYNTHESIS OF NITRATE REDUCTASE. Nature. 1964 Jan 4;201:58–60. doi: 10.1038/201058a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg C., Casse-Delbart F., Dusha I., David M., Boucher C. Megaplasmids in the plant-associated bacteria Rhizobium meliloti and Pseudomonas solanacearum. J Bacteriol. 1982 Apr;150(1):402–406. doi: 10.1128/jb.150.1.402-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römermann D., Friedrich B. Denitrification by Alcaligenes eutrophus is plasmid dependent. J Bacteriol. 1985 May;162(2):852–854. doi: 10.1128/jb.162.2.852-854.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Urban M., Friedrich B. Mutagenesis of Alcaligenes eutrophus by insertion of the drug-resistance transposon Tn5. Arch Microbiol. 1982 May;131(3):203–207. doi: 10.1007/BF00405879. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. D-ribulose 1,5-diphosphate carboxylase from Rhodospirillum rubrum. I. Levels, purification, and effects of metallic ions. J Biol Chem. 1974 Jun 10;249(11):3453–3458. [PubMed] [Google Scholar]
- Toghrol F., Southerland W. M. Purification of Thiobacillus novellus sulfite oxidase. Evidence for the presence of heme and molybdenum. J Biol Chem. 1983 Jun 10;258(11):6762–6766. [PubMed] [Google Scholar]





