Abstract
The isolation of a glycoprotein from vegetative cells of Myxococcus xanthus is reported. The protein, abbreviated VGP, was first identified during a survey of surface proteins as a major protein that could be radioiodinated in vegetative, but not developing, cells (P.Y. Maeba, J. Bacteriol. 155:1033-1041, 1983). The protein was extracted from membranes with Triton X-100 and subsequently purified by DEAE-cellulose chromatography, chromatofocusing, and gel filtration. The protein has an Mr of approximately 74,000 as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and an isoelectric point of 3.2 to 3.3. The carbohydrate moiety which made up approximately 13.5% of the weight of the VGP comprised primarily neutral sugars and smaller amounts of hexosamines and uronic acids. The amino acid content revealed no unusual features, but analysis by the method of Barrantes (F. Barrantes, Biochem. Biophys. Res. Commun. 62:407-414, 1975) indicated it is likely a peripheral membrane protein. The protein makes up approximately 1% of the total cell protein and is a prominent surface structure. Because glycoproteins have been implicated in cellular interactions in a number of systems, the VGP may play an important role in the social behavior exhibited by M. xanthus.
Full text
PDF




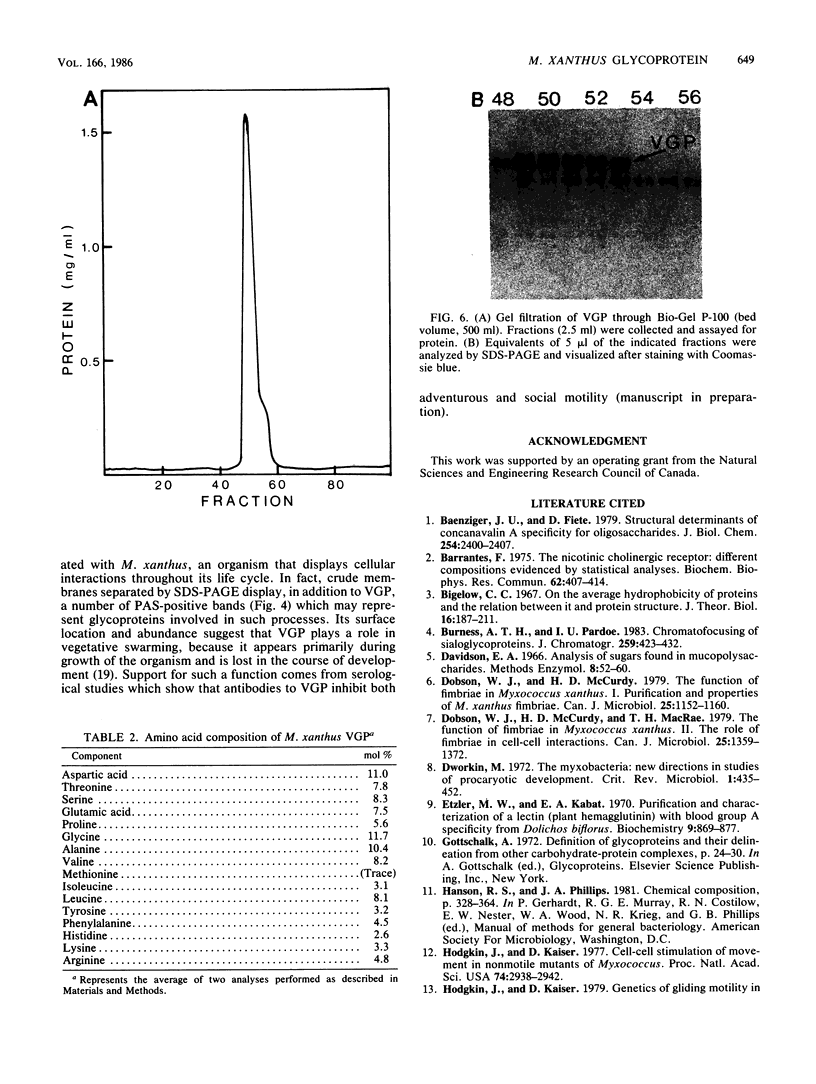

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J. U., Fiete D. Structural determinants of concanavalin A specificity for oligosaccharides. J Biol Chem. 1979 Apr 10;254(7):2400–2407. [PubMed] [Google Scholar]
- Barrantes F. J. The nicotinic cholinergic receptor : different compositions evidenced by statistical analysis. Biochem Biophys Res Commun. 1975 Jan 20;62(2):407–414. doi: 10.1016/s0006-291x(75)80153-7. [DOI] [PubMed] [Google Scholar]
- Bigelow C. C. On the average hydrophobicity of proteins and the relation between it and protein structure. J Theor Biol. 1967 Aug;16(2):187–211. doi: 10.1016/0022-5193(67)90004-5. [DOI] [PubMed] [Google Scholar]
- Burness A. T., Pardoe I. U. Chromatofocusing of sialoglycoproteins. J Chromatogr. 1983 Apr 15;259(3):423–432. doi: 10.1016/s0021-9673(01)88030-4. [DOI] [PubMed] [Google Scholar]
- Dobson W. J., McCurdy H. D., MacRae T. H. The function of fimbriae in Myxococcus xanthus. II. The role of fimbriae in cell-cell interactions. Can J Microbiol. 1979 Dec;25(12):1359–1372. doi: 10.1139/m79-214. [DOI] [PubMed] [Google Scholar]
- Dobson W. J., McCurdy H. D. The function of fimbriae in Myxococcus xanthus. I. Purification and properties of M. xanthus fimbriae. Can J Microbiol. 1979 Oct;25(10):1152–1160. doi: 10.1139/m79-179. [DOI] [PubMed] [Google Scholar]
- Etzler M. E., Kabat E. A. Purification and characterization of a lectin (plant hemagglutinin) with blood group A specificity from Dolichos biflorus. Biochemistry. 1970 Feb 17;9(4):869–877. doi: 10.1021/bi00806a022. [DOI] [PubMed] [Google Scholar]
- Hodgkin J., Kaiser D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- Kaiser D., Manoil C., Dworkin M. Myxobacteria: cell interactions, genetics, and development. Annu Rev Microbiol. 1979;33:595–639. doi: 10.1146/annurev.mi.33.100179.003115. [DOI] [PubMed] [Google Scholar]
- Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeba P. Y. Iodination of Myxococcus xanthus during development. J Bacteriol. 1983 Sep;155(3):1033–1041. doi: 10.1128/jb.155.3.1033-1041.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Corre C. Fractionation of the multiple forms of bovine gastric aspartic proteases by chromatofocusing. Anal Biochem. 1984 Dec;143(2):256–264. doi: 10.1016/0003-2697(84)90661-4. [DOI] [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Purification and characterization of a prokaryotic glucoprotein from the cell envelope of Halobacterium salinarium. J Biol Chem. 1976 Apr 10;251(7):2005–2014. [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L., Watson S. W. Protein and carbohydrate composition of the cell envelope of Halobacterium salinarium. J Bacteriol. 1974 Nov;120(2):945–954. doi: 10.1128/jb.120.2.945-954.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Dworkin M. Separation and properties of the cytoplasmic and outer membranes of vegetative cells of Myxococcus xanthus. J Bacteriol. 1980 Feb;141(2):914–927. doi: 10.1128/jb.141.2.914-927.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]







