Abstract
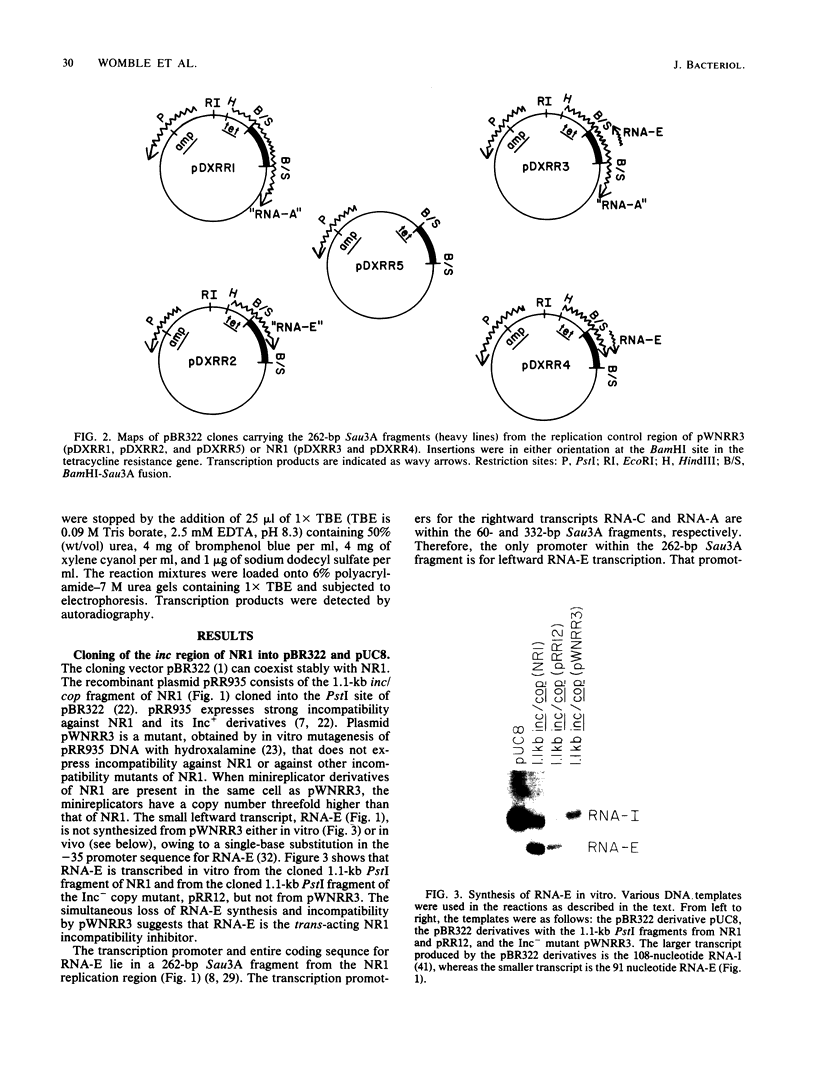
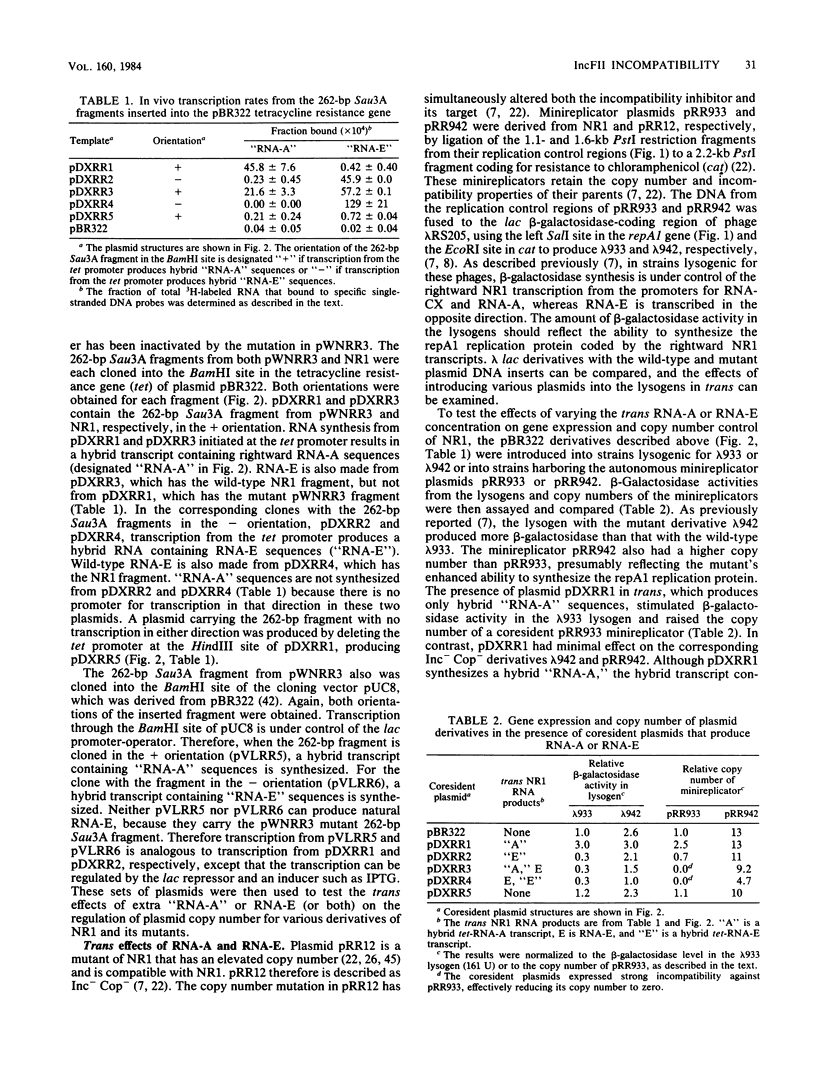
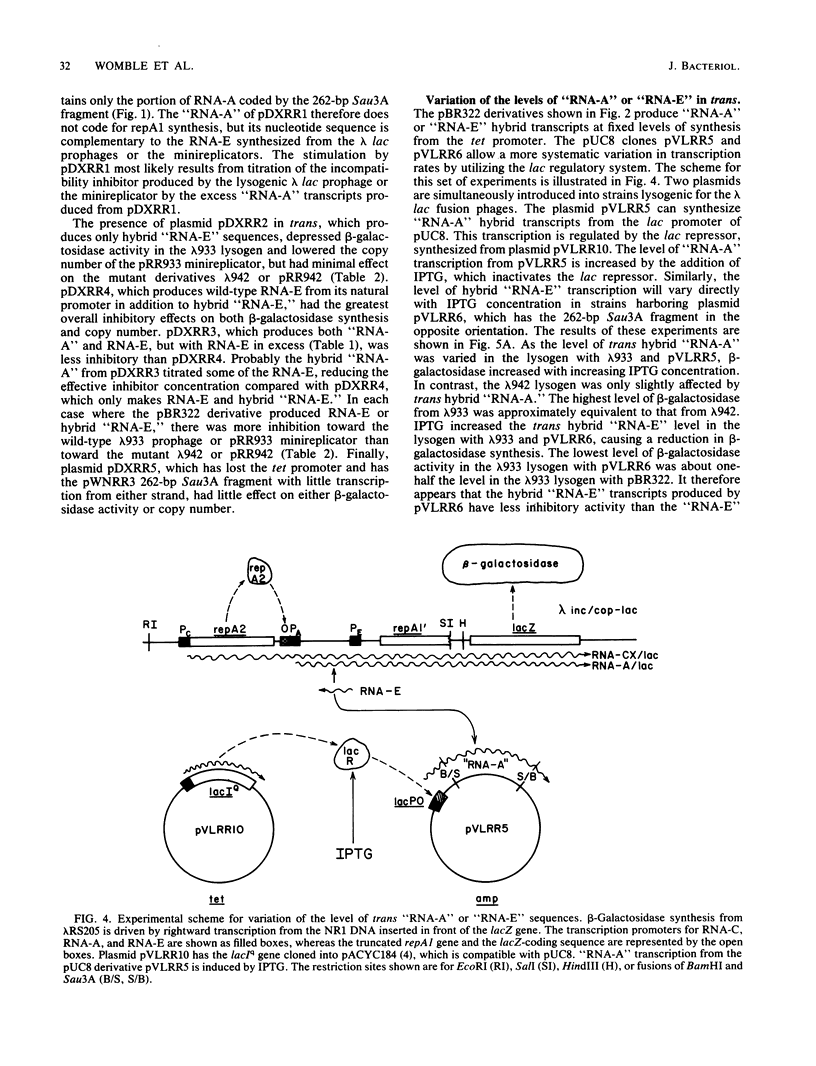
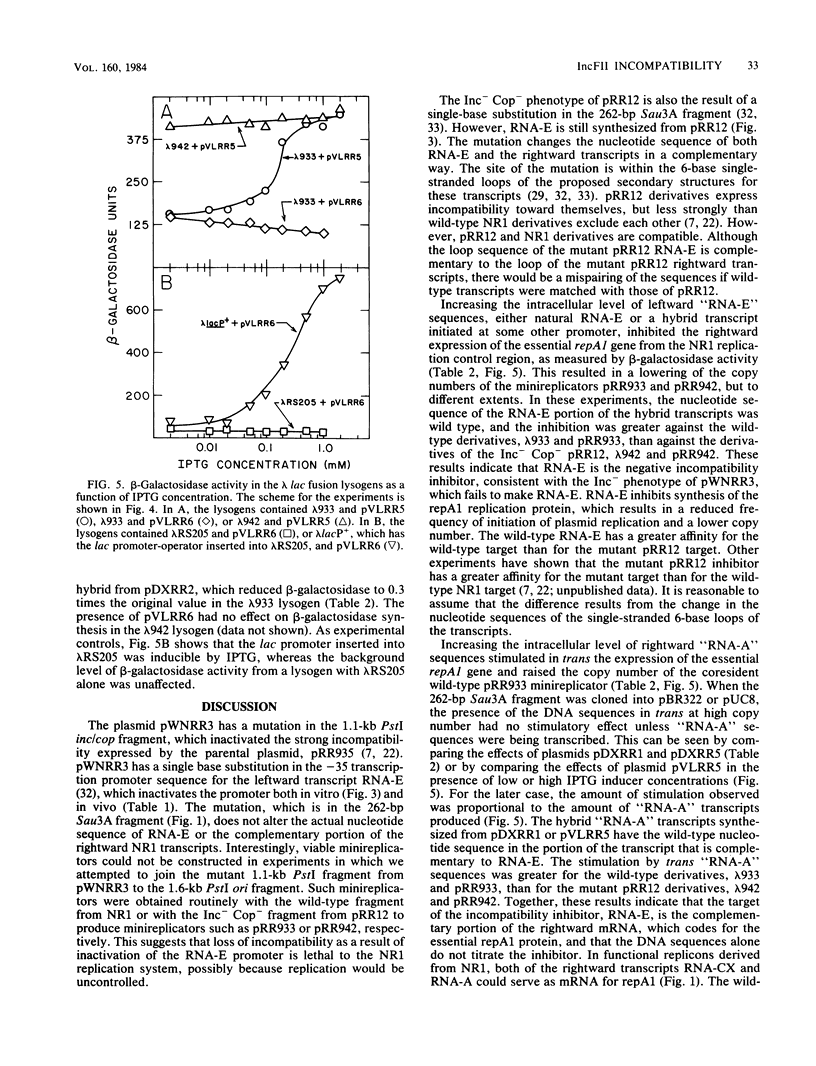
The region of DNA coding for incompatibility (inc) and copy number control (cop) of the IncFII plasmid NR1 is transcribed in both the rightward and leftward directions. The rightward transcripts serve as mRNA for the repA1 protein, which is required for replication. A small, 91-base leftward transcript is synthesized from the opposite DNA strand and is complementary to a portion of the rightward mRNA near its 5' end. A 262-base-pair Sau3A restriction fragment that encodes the small leftward transcript, but does not include the rightward transcription promoters, was cloned into the vector pBR322 or pUC8. The same fragment was cloned from an Inc- mutant of NR1 that does not make the small leftward transcript. Transcription through the cloned fragments in these derivatives was under control of the tetracycline resistance gene in pBR322 or the lac promoter-operator in pUC8. In one orientation of the inserted DNA, a hybrid transcript containing rightward NR1 RNA sequences was synthesized. In the other orientation, a hybrid transcript containing leftward NR1 RNA sequences was synthesized. These plasmids were used to vary the intracellular levels of the rightward or leftward NR1 RNA transcripts and to test their effects in trans on various coresident derivatives of NR1. An excess of rightward NR1 RNA in trans stimulated expression of the essential repA1 gene and caused an increase in the copy number of a coresident NR1 plasmid. An excess of leftward NR1 RNA in trans inhibited the expression of the repA1 gene and lowered the coresident NR1 copy number, thereby causing incompatibility. A pBR322 derivative with no transcription through the cloned NR1 DNA had no effect in trans. These results suggest that the small leftward transcript is the incompatibility inhibitor of NR1 and that its target is the complementary portion of the rightward mRNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brady G., Frey J., Danbara H., Timmis K. N. Replication control mutations of plasmid R6-5 and their effects on interactions of the RNA-I control element with its target. J Bacteriol. 1983 Apr;154(1):429–436. doi: 10.1128/jb.154.1.429-436.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner M. E., Jaskunas S. R. Identification of polypeptides encoded by the replication of resistance factor R100. J Mol Biol. 1982 Jul 25;159(1):35–55. doi: 10.1016/0022-2836(82)90030-4. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbara H., Brady G., Timmis J. K., Timmis K. N. Regulation of DNA replication: "target" determinant of the replication control elements of plasmid R6-5 lies within a control element gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4699–4703. doi: 10.1073/pnas.78.8.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A. M., Rownd R. H. The incompatibility product of IncFII R plasmid NR1 controls gene expression in the plasmid replication region. J Bacteriol. 1982 Nov;152(2):829–839. doi: 10.1128/jb.152.2.829-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forchhammer J., Jackson E. N., Yanofsky C. Different half-lives of messenger RNA corresponding to different segments of the tryptophan operon of Escherichia coli. J Mol Biol. 1972 Nov 28;71(3):687–699. doi: 10.1016/s0022-2836(72)80032-9. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Guarente L., Lauer G., Roberts T. M., Ptashne M. Improved methods for maximizing expression of a cloned gene: a bacterium that synthesizes rabbit beta-globin. Cell. 1980 Jun;20(2):543–553. doi: 10.1016/0092-8674(80)90640-6. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Light J., Molin S. Post-transcriptional control of expression of the repA gene of plasmid R1 mediated by a small RNA molecule. EMBO J. 1983;2(1):93–98. doi: 10.1002/j.1460-2075.1983.tb01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light J., Molin S. Replication control functions of plasmid R1 act as inhibitors of expression of a gene required for replication. Mol Gen Genet. 1981;184(1):56–61. doi: 10.1007/BF00271195. [DOI] [PubMed] [Google Scholar]
- Light J., Molin S. The sites of action of the two copy number control functions of plasmid R1. Mol Gen Genet. 1982;187(3):486–493. doi: 10.1007/BF00332633. [DOI] [PubMed] [Google Scholar]
- Liu C. P., Churchward G., Caro L. The repA2 gene of the plasmid R100.1 encodes a repressor of plasmid replication. Plasmid. 1983 Sep;10(2):148–155. doi: 10.1016/0147-619x(83)90067-7. [DOI] [PubMed] [Google Scholar]
- Lurz R., Danbara H., Rückert B., Timmis K. N. Plasmid replication functions. VII. Electron microscopic localization of RNA polymerase binding sites in the replication control region of plasmid R6-5. Mol Gen Genet. 1981;183(3):490–496. doi: 10.1007/BF00268770. [DOI] [PubMed] [Google Scholar]
- Masai H., Kaziro Y., Arai K. Definition of oriR, the minimum DNA segment essential for initiation of R1 plasmid replication in vitro. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6814–6818. doi: 10.1073/pnas.80.22.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Miki T., Easton A. M., Rownd R. H. Cloning of replication, incompatibility, and stability functions of R plasmid NR1. J Bacteriol. 1980 Jan;141(1):87–99. doi: 10.1128/jb.141.1.87-99.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin S., Nordström K. Control of plasmid R1 replication: functions involved in replication, copy number control, incompatibility, and switch-off of replication. J Bacteriol. 1980 Jan;141(1):111–120. doi: 10.1128/jb.141.1.111-120.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin S., Stougaard P., Light J., Nordström M., Nordström K. Isolation and characterization of new copy mutants of plasmid R1, and identification of a polypeptide involved in copy number control. Mol Gen Genet. 1981;181(1):123–130. doi: 10.1007/BF00339015. [DOI] [PubMed] [Google Scholar]
- Morris C. F., Hashimoto H., Mickel S., Rownd R. Round of replication mutant of a drug resistance factor. J Bacteriol. 1974 Jun;118(3):855–866. doi: 10.1128/jb.118.3.855-866.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo E., Feingold J., Ohtsubo H., Mickel S., Bauer W. Unidirectional replication in Escherichia coli of three small plasmids derived from R factor R12. Plasmid. 1977 Nov;1(1):8–18. doi: 10.1016/0147-619x(77)90004-x. [DOI] [PubMed] [Google Scholar]
- Rosen J., Ryder T., Inokuchi H., Ohtsubo H., Ohtsubo E. Genes and sites involved in replication and incompatibility of an R100 plasmid derivative based on nucleotide sequence analysis. Mol Gen Genet. 1980;179(3):527–537. doi: 10.1007/BF00271742. [DOI] [PubMed] [Google Scholar]
- Rosen J., Ryder T., Ohtsubo H., Ohtsubo E. Role of RNA transcripts in replication incompatibility and copy number control in antibiotic resistance plasmid derivatives. Nature. 1981 Apr 30;290(5809):794–797. doi: 10.1038/290794a0. [DOI] [PubMed] [Google Scholar]
- Rownd R., Nakaya R., Nakamura A. Molecular nature of the drug-resistance factors of the Enterobacteriaceae. J Mol Biol. 1966 Jun;17(2):376–393. doi: 10.1016/s0022-2836(66)80149-3. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Translational control of IS10 transposition. Cell. 1983 Sep;34(2):683–691. doi: 10.1016/0092-8674(83)90401-4. [DOI] [PubMed] [Google Scholar]
- Stougaard P., Molin S., Nordström K., Hansen F. G. The nucleotide sequence of the replication control region of the resistance plasmid R1drd-19. Mol Gen Genet. 1981;181(1):116–122. doi: 10.1007/BF00339014. [DOI] [PubMed] [Google Scholar]
- Stougaard P., Molin S., Nordström K. RNAs involved in copy-number control and incompatibility of plasmid R1. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6008–6012. doi: 10.1073/pnas.78.10.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synenki R. M., Nordheim A., Timmis K. N. Plasmid replication functions. III. Origin and direction of replication of a "mini" plasmid derived from R6-5. Mol Gen Genet. 1979 Jan 5;168(1):27–36. doi: 10.1007/BF00267930. [DOI] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N. Structural and functional analysis of cloned DNA segments containing the replication and incompatibility regions of a miniplasmid derived from a copy number mutant of NR1. J Bacteriol. 1979 Jan;137(1):92–104. doi: 10.1128/jb.137.1.92-104.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. N., Andrés I., Slocombe P. M. Plasmid incompatibility: cloning analysis of an incFII determinant of R6-5. Nature. 1978 May 4;273(5657):27–32. doi: 10.1038/273027a0. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Itoh T., Selzer G., Som T. Inhibition of ColE1 RNA primer formation by a plasmid-specified small RNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1421–1425. doi: 10.1073/pnas.78.3.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Winkler M. E., Mullis K., Barnett J., Stroynowski I., Yanofsky C. Transcription termination at the tryptophan operon attenuator is decreased in vitro by an oligomer complementary to a segment of the leader transcript. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2181–2185. doi: 10.1073/pnas.79.7.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble D. D., Taylor D. P., Rownd R. H. Method for obtaining more-accurate covalently closed circular plasmid-to-chromosome ratios from bacterial lysates by dye-buoyant density centrifugation. J Bacteriol. 1977 Apr;130(1):148–153. doi: 10.1128/jb.130.1.148-153.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Nomura M. Feedback regulation of ribosomal protein synthesis in E. coli: localization of the mRNA target sites for repressor action of ribosomal protein L1. Cell. 1981 Apr;24(1):243–249. doi: 10.1016/0092-8674(81)90520-1. [DOI] [PubMed] [Google Scholar]
- von Hippel P. H., Kowalczykowski S. C., Lonberg N., Newport J. W., Paul L. S., Stormo G. D., Gold L. Autoregulation of gene expression. Quantitative evaluation of the expression and function of the bacteriophage T4 gene 32 (single-stranded DNA binding) protein system. J Mol Biol. 1982 Dec 25;162(4):795–818. doi: 10.1016/0022-2836(82)90548-4. [DOI] [PubMed] [Google Scholar]



