Abstract
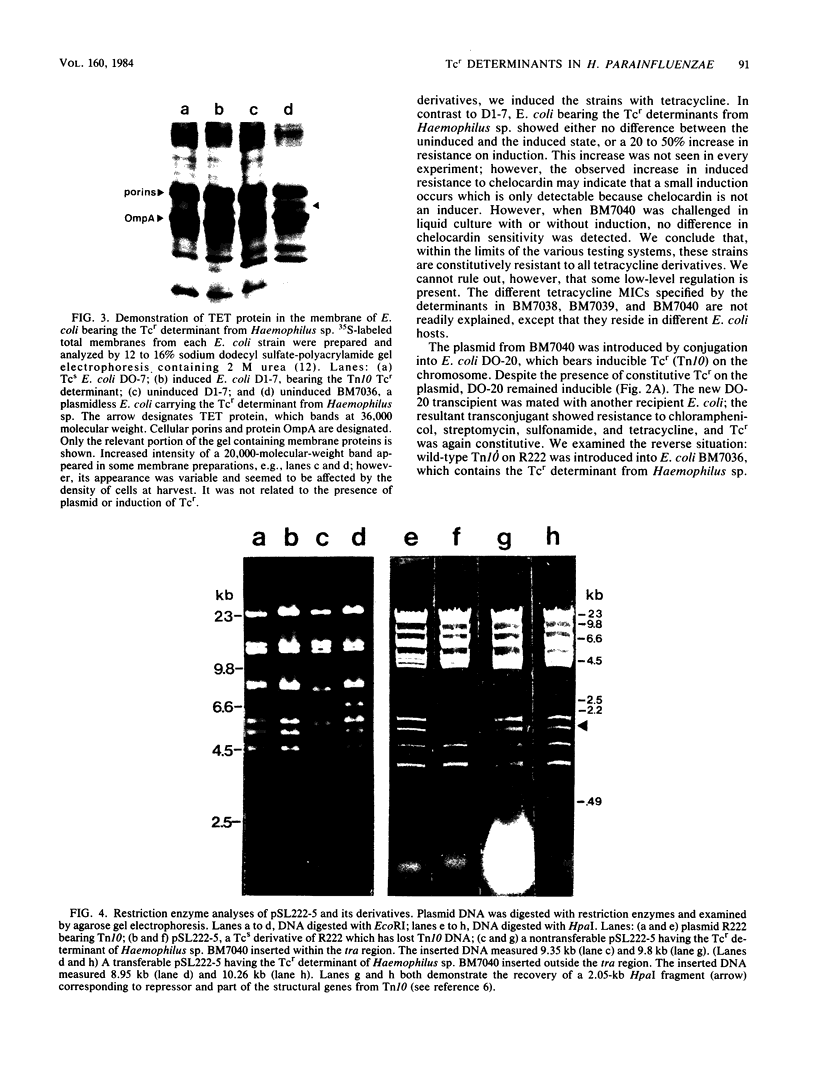
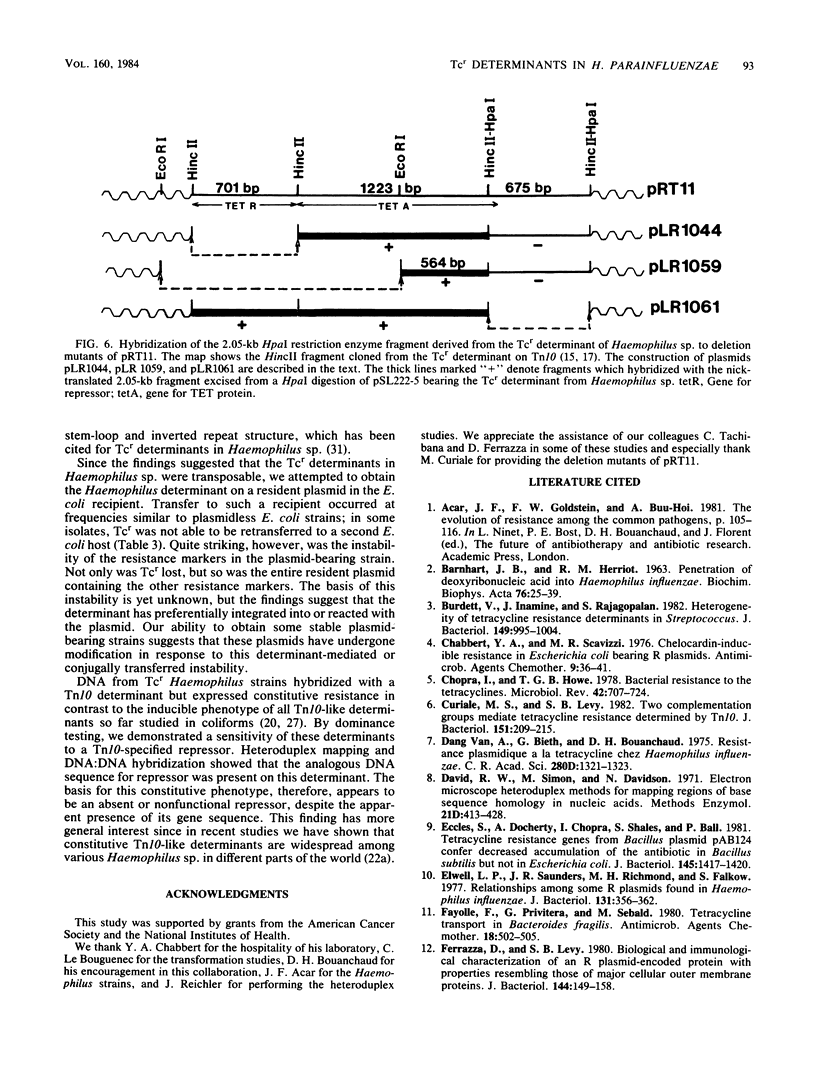
Tetracycline resistance (Tcr) determinants from three different strains of Haemophilus parainfluenzae expressed 10-fold higher levels of resistance when mated into Escherichia coli. No plasmid was found in any of the E. coli recipients, even in matings in which a plasmid was identified in the donor Haemophilus sp. The Tcr determinant from Haemophilus sp. caused instability of resident plasmids in the recipient E. coli: all plasmids were lost within 30 generations in antibiotic-free media. However, by serial subculture in antibiotics, stable resident plasmids were obtained which carried the Tcr determinant from Haemophilus sp. and were transferable by conjugation and transformation among E. coli strains. All Haemophilus determinants hybridized with a probe for the Tcr determinant on Tn10, which bears inducible Tcr. However, Haemophilus determinants were constitutively resistant to tetracycline in the Haemophilus donors and in the E. coli recipients. This constitutive expression was recessive to wild-type Tn10 in the same cell, indicating that the constitutive phenotype resulted from the absence of an active repressor. Restrictive enzyme analysis of various E. coli plasmid derivatives bearing a Tcr determinant from Haemophilus sp. demonstrated that the inserted DNA was of similar size (8.95 to 9.35 kilobases), close to that of Tn10. Heteroduplex analysis and DNA:DNA hybridization confirmed that the Tcr determinant from Haemophilus sp. had greater than 90% homology with the Tn10 determinant, including the DNA sequence for the repressor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNHART B. J., HERRIOTT R. M. PENETRATION OF DEOXYRIBONUCLEIC ACID INTO HEMOPHILUS INFLUENZAE. Biochim Biophys Acta. 1963 Sep 17;76:25–39. [PubMed] [Google Scholar]
- Burdett V., Inamine J., Rajagopalan S. Heterogeneity of tetracycline resistance determinants in Streptococcus. J Bacteriol. 1982 Mar;149(3):995–1004. doi: 10.1128/jb.149.3.995-1004.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabbert Y. A., Scavizzi M. R. Chelocardin-inducible resistance in Escherichia coli bearing R plasmids. Antimicrob Agents Chemother. 1976 Jan;9(1):36–41. doi: 10.1128/aac.9.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I., Howe T. G. Bacterial resistance to the tetracyclines. Microbiol Rev. 1978 Dec;42(4):707–724. doi: 10.1128/mr.42.4.707-724.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiale M. S., Levy S. B. Two complementation groups mediate tetracycline resistance determined by Tn10. J Bacteriol. 1982 Jul;151(1):209–215. doi: 10.1128/jb.151.1.209-215.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles S., Docherty A., Chopra I., Shales S., Ball P. Tetracycline resistance genes from Bacillus plasmid pAB124 confer decreased accumulation of the antibiotic in Bacillus subtilis but not in Escherichia coli. J Bacteriol. 1981 Mar;145(3):1417–1420. doi: 10.1128/jb.145.3.1417-1420.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., Saunders J. R., Richmond M. H., Falkow S. Relationships among some R plasmids found in Haemophilus influenzae. J Bacteriol. 1977 Jul;131(1):356–362. doi: 10.1128/jb.131.1.356-362.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayolle F., Privitera G., Sebald M. Tetracycline transport in Bacteroides fragilis. Antimicrob Agents Chemother. 1980 Oct;18(4):502–505. doi: 10.1128/aac.18.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrazza D., Levy S. B. Biochemical and immunological characterization of an R plasmid-encoded protein with properties resembling those of major cellular outer membrane proteins. J Bacteriol. 1980 Oct;144(1):149–158. doi: 10.1128/jb.144.1.149-158.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Howe T. G., Richmond K. M. Translocation of the tetracycline resistance determinant from R100-1 to the Escherichia coli K-12 chromosome. J Bacteriol. 1975 Dec;124(3):1153–1158. doi: 10.1128/jb.124.3.1153-1158.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein F. W., Boisivon A., Leclerc P., Acar J. F. Sensibilité d'Hemophilus sp. aux antibiotiques. transfert de résistance à Escherichia coli. Pathol Biol (Paris) 1977 May;25(5):323–332. [PubMed] [Google Scholar]
- Hillen W., Schollmeier K. Nucleotide sequence of the Tn10 encoded tetracycline resistance gene. Nucleic Acids Res. 1983 Jan 25;11(2):525–539. doi: 10.1093/nar/11.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan J. E., Kline B. C., Levy S. B. Regions on the F plasmid which affect plasmid maintenance and the ability to segregate into Escherichia coli minicells. Plasmid. 1982 Jul;8(1):36–44. doi: 10.1016/0147-619x(82)90039-7. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Reznikoff W. S. Organization of structural and regulatory genes that mediate tetracycline resistance in transposon Tn10. J Bacteriol. 1979 Jun;138(3):705–714. doi: 10.1128/jb.138.3.705-714.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulfers P. M., Laufs R., Jahn G. Molecular properties of transmissible R factors of Haemophilus influenzae determing tetracycline resistance. J Gen Microbiol. 1978 Apr;105(2):243–252. doi: 10.1099/00221287-105-2-243. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- Marshall B., Roberts M., Smith A., Levy S. B. Homogeneity of transferable tetracycline-resistance determinants in Haemophilus species. J Infect Dis. 1984 Jun;149(6):1028–1029. doi: 10.1093/infdis/149.6.1028. [DOI] [PubMed] [Google Scholar]
- Marshall B., Schluederberg S., Tachibana C., Levy S. B. Survival and transfer in the human gut of poorly mobilizable (pBR322) and of transferable plasmids from the same carrier E. coli. Gene. 1981 Aug;14(3):145–154. doi: 10.1016/0378-1119(81)90110-4. [DOI] [PubMed] [Google Scholar]
- Marshall B., Tachibana C., Levy S. B. Frequency of tetracycline resistance determinant classes among lactose-fermenting coliforms. Antimicrob Agents Chemother. 1983 Dec;24(6):835–840. doi: 10.1128/aac.24.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L. M., Cullinane J. C., Levy S. B. Transport of the lipophilic analog minocycline differs from that of tetracycline in susceptible and resistant Escherichia coli strains. Antimicrob Agents Chemother. 1982 Nov;22(5):791–799. doi: 10.1128/aac.22.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez B., Tachibana C., Levy S. B. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980 Mar;3(2):99–108. doi: 10.1016/0147-619x(80)90101-8. [DOI] [PubMed] [Google Scholar]
- Ptashne K., Cohen S. N. Occurrence of insertion sequence (IS) regions on plasmid deoxyribonucleic acid as direct and inverted nucleotide sequence duplications. J Bacteriol. 1975 May;122(2):776–781. doi: 10.1128/jb.122.2.776-781.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Hazum S., Guild W. R. Homology among tet determinants in conjugative elements of streptococci. J Bacteriol. 1981 Oct;148(1):232–240. doi: 10.1128/jb.148.1.232-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky D., Krawitz T., Zaidenzaig Y., Abramova N. Inducible resistance to tetracycline in Staphylococcus aureus. J Gen Microbiol. 1970 Aug;62(3):341–349. doi: 10.1099/00221287-62-3-341. [DOI] [PubMed] [Google Scholar]
- Spies T., Laufs R., Riess F. C. Amplification of resistance genes in Haemophilus influenzae plasmids. J Bacteriol. 1983 Aug;155(2):839–846. doi: 10.1128/jb.155.2.839-846.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Chromosomally integrated conjugative plasmids are common in antibiotic-resistant Haemophilus influenzae. J Bacteriol. 1980 Jun;142(3):925–930. doi: 10.1128/jb.142.3.925-930.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., De Grandis S. A., Karmali M. A., Fleming P. C. Transmissible plasmids from Campylobacter jejuni. Antimicrob Agents Chemother. 1981 May;19(5):831–835. doi: 10.1128/aac.19.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van A. D., Bieth G., Bouanchaud D. H. Résistance plasmidique à la tétracycline chez Haemophilus influenzae. C R Acad Sci Hebd Seances Acad Sci D. 1975 Mar 10;280(10):1321–1323. [PubMed] [Google Scholar]
- van Klingeren B., van Embden J. D., Dessens-Kroon M. Plasmid-mediated chloramphenicol resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1977 Mar;11(3):383–387. doi: 10.1128/aac.11.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]





