Abstract
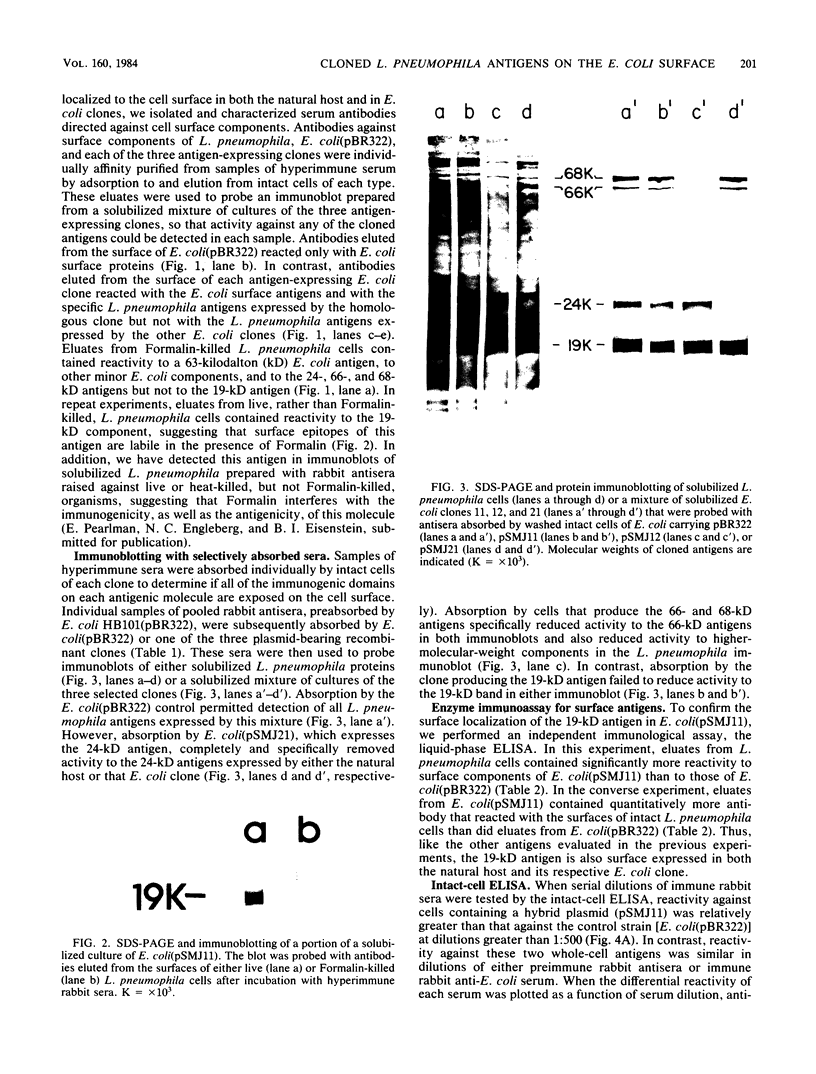
Escherichia coli clones that express Legionella pneumophila antigens were isolated from a plasmid genomic library, and their antigens were characterized by immunoblotting with rabbit anti-L. pneumophila sera. Because previous studies of L. pneumophila antigens by whole-cell radioimmunoprecipitation suggested that comigrating native antigens were surface localized, we conducted experiments to determine if the cloned antigens were surface expressed in E. coli. Aliquots of antisera were absorbed by intact cells of three representative antigen-producing E. coli clones, and surface-bound antibodies were acid eluted from the intact cells. Immunoblots made with selectively absorbed antisera and eluted antibodies confirmed that reactivity to the homologous cloned antigens could be specifically absorbed from the antisera and then eluted from the cells, demonstrating a surface (antibody-accessible) localization in the cloned state. Antibodies eluted from the surface of an E. coli clone that expressed a 19-kilodalton antigen reacted with the surface of L. pneumophila in a liquid-phase, whole-cell enzyme-linked immunosorbent assay. In addition, intact cells of this clone were used in an enzyme-linked immunosorbent assay to detect serum antibody. E. coli cells that express foreign antigens on their surfaces can be used to develop antigen-specific immunoassays and to affinity purify monospecific antibodies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano K., Williams J. C. Peptidoglycan of Legionella pneumophila: apparent resistance to lysozyme hydrolysis correlates with a high degree of peptide cross-linking. J Bacteriol. 1983 Jan;153(1):520–526. doi: 10.1128/jb.153.1.520-526.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman P. W., Dowbenko D., Lasky L. A., Simonsen C. C. Detection of antibodies to herpes simplex virus with a continuous cell line expressing cloned glycoprotein D. Science. 1983 Nov 4;222(4623):524–527. doi: 10.1126/science.6312563. [DOI] [PubMed] [Google Scholar]
- Engleberg N. C., Drutz D. J., Eisenstein B. I. Cloning and expression of Legionella pneumophila antigens in Escherichia coli. Infect Immun. 1984 May;44(2):222–227. doi: 10.1128/iai.44.2.222-227.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Manjula B. N., Scott J. R. Streptococcal M6 protein expressed in Escherichia coli. Localization, purification, and comparison with streptococcal-derived M protein. J Exp Med. 1984 Apr 1;159(4):1083–1095. doi: 10.1084/jem.159.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey J. M., Gill R. E., Falkow S. Genetic and biochemical analysis of gonococcal IgA1 protease: cloning in Escherichia coli and construction of mutants of gonococci that fail to produce the activity. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7881–7885. doi: 10.1073/pnas.79.24.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leong D., Coleman K. D., Murphy J. R. Cloned fragment A of diphtheria toxin is expressed and secreted into the periplasmic space of Escherichia coli K12. Science. 1983 Apr 29;220(4596):515–517. doi: 10.1126/science.6403984. [DOI] [PubMed] [Google Scholar]
- Lory S., Tai P. C. Characterization of the phospholipase C gene of Pseudomonas aeruginosa cloned in Escherichia coli. Gene. 1983 Apr;22(1):95–101. doi: 10.1016/0378-1119(83)90068-9. [DOI] [PubMed] [Google Scholar]
- Meyer R. D. Legionella infections: a review of five years of research. Rev Infect Dis. 1983 Mar-Apr;5(2):258–278. doi: 10.1093/clinids/5.2.258. [DOI] [PubMed] [Google Scholar]
- Pearson G. D., Mekalanos J. J. Molecular cloning of Vibrio cholerae enterotoxin genes in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 May;79(9):2976–2980. doi: 10.1073/pnas.79.9.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell B. K., Clegg S. Construction and expression of recombinant plasmids encoding type 1 fimbriae of a urinary Klebsiella pneumoniae isolate. Infect Immun. 1983 Mar;39(3):1122–1127. doi: 10.1128/iai.39.3.1122-1127.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvas M. O., Palva I. A. The penicillinase of Bacillus licheniformis is an outer membrane protein in Escherichia coli. J Bacteriol. 1983 Aug;155(2):657–663. doi: 10.1128/jb.155.2.657-663.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. H., Schalla W. O., Arko R. J., Bullard J. C., Feeley J. C. Immunochemical, serologic, and immunologic properties of major antigens isolated from the Legionnaires' disease bacterium. Observations bearing on the feasibility of a vaccine. Ann Intern Med. 1979 Apr;90(4):634–638. doi: 10.7326/0003-4819-90-4-634. [DOI] [PubMed] [Google Scholar]





